Osteoprotegerin Induces Apoptosis of Osteoclasts and Osteoclast Precursor Cells via the Fas/Fas Ligand Pathway
- PMID: 26571489
- PMCID: PMC4646684
- DOI: 10.1371/journal.pone.0142519
Osteoprotegerin Induces Apoptosis of Osteoclasts and Osteoclast Precursor Cells via the Fas/Fas Ligand Pathway
Abstract
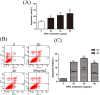
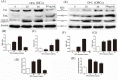
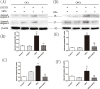
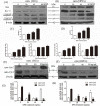
Osteoprotegerin (OPG) is known to inhibit differentiation and activation of osteoclasts (OCs) by functioning as a decoy receptor blocking interactions between RANK and RANKL. However, the exact role of OPG in the survival/apoptosis of OCs remains unclear. OPG caused increased rates of apoptosis of both OCs and osteoclast precursor cells (OPCs). The expression of Fas and activated caspase-8 was increased by both 20 ng/mL and 40 ng/mL of OPG, but was markedly decreased at 80 ng/mL. Interestingly, we noted that while levels of Fas ligand (FasL) increased with increasing doses of OPG, the soluble form of FasL in the supernatant decreased. The results of a co-immunoprecipitation assay suggested that the decrease of sFasL might be caused by the binding of OPG. This would block the inhibition of the apoptosis of OCs and OPCs. Furthermore, changes in expression levels of Bax/Bcl-2, cleaved-caspase-9, cleaved-caspased-3 and the translocation of cytochrome c, illustrated that OPG induced apoptosis of OCs and OPCs via the classic Fas/FasL apoptosis pathway, and was mediated by mitochondria. Altogether, our results demonstrate that OPG induces OCs and OPCs apoptosis partly by the Fas/FasL signaling pathway.
Conflict of interest statement
Figures

Similar articles
-
Osteoprotegerin decreases human osteoclast apoptosis by inhibiting the TRAIL pathway.J Cell Physiol. 2008 Aug;216(2):536-42. doi: 10.1002/jcp.21430. J Cell Physiol. 2008. PMID: 18338379
-
Osteoblast-induced osteoclast apoptosis by fas ligand/FAS pathway is required for maintenance of bone mass.Cell Death Differ. 2015 Oct;22(10):1654-64. doi: 10.1038/cdd.2015.14. Epub 2015 Mar 6. Cell Death Differ. 2015. PMID: 25744024 Free PMC article.
-
Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system.Endocrinology. 2005 Apr;146(4):1991-8. doi: 10.1210/en.2004-1167. Epub 2004 Dec 23. Endocrinology. 2005. PMID: 15618359
-
Osteoprotegerin and inflammation.Eur Cytokine Netw. 2002 Apr-Jun;13(2):144-53. Eur Cytokine Netw. 2002. PMID: 12101070 Review.
-
RANK/RANKL/OPG role in distraction osteogenesis.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010 May;109(5):679-86. doi: 10.1016/j.tripleo.2009.10.042. Epub 2010 Feb 16. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010. PMID: 20163972 Review.
Cited by
-
Osteoclast Recycling and the Rebound Phenomenon Following Denosumab Discontinuation.Curr Osteoporos Rep. 2022 Dec;20(6):505-515. doi: 10.1007/s11914-022-00756-5. Epub 2022 Oct 6. Curr Osteoporos Rep. 2022. PMID: 36201122 Free PMC article. Review.
-
New Insights Into Osteoclast Biology.JBMR Plus. 2021 Aug 30;5(9):e10539. doi: 10.1002/jbm4.10539. eCollection 2021 Sep. JBMR Plus. 2021. PMID: 34532619 Free PMC article. Review.
-
Validity of Osteoprotegerin and Receptor Activator of NF-κB Ligand for the Detection of Bone Metastasis in Breast Cancer.Oncol Res. 2017 Apr 14;25(4):641-650. doi: 10.3727/096504016X14768398678750. Epub 2016 Oct 26. Oncol Res. 2017. PMID: 27983911 Free PMC article.
-
Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis.Cell Prolif. 2021 Jan;54(1):e12956. doi: 10.1111/cpr.12956. Epub 2020 Nov 18. Cell Prolif. 2021. PMID: 33210341 Free PMC article. Review.
-
Correlation between osteoprotegerin and coronary artery calcification in diabetic subjects: a systematic review of observational studies.BMC Cardiovasc Disord. 2023 Feb 21;23(1):96. doi: 10.1186/s12872-023-03123-z. BMC Cardiovasc Disord. 2023. PMID: 36809976 Free PMC article.
References
-
- Baud'huin M, Duplomb L, Teletchea S, Lamoureux F, Ruiz-Velasco C, Maillasson M, et al. Osteoprotegerin: Multiple partners for multiple functions. Cytokine Growth F R 2013. - PubMed
-
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–19. - PubMed
-
- Tsuda E, Goto M, Mochizuki S-i, Yano K, Kobayashi F, Morinaga T, et al. Isolation of a Novel Cytokine from Human Fibroblasts That Specifically Inhibits Osteoclastogenesis. Biochem Biophys Res Commun 1997;234(1):137–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

