TDP-43 Inhibits NF-κB Activity by Blocking p65 Nuclear Translocation
- PMID: 26571498
- PMCID: PMC4646651
- DOI: 10.1371/journal.pone.0142296
TDP-43 Inhibits NF-κB Activity by Blocking p65 Nuclear Translocation
Abstract
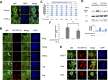
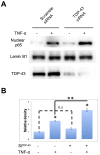
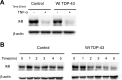
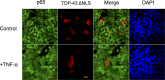
TDP-43 (TAR DNA binding protein 43) is a heterogeneous nuclear ribonucleoprotein (hnRNP) that has been found to play an important role in neurodegenerative diseases. TDP-43's involvement in nuclear factor-kappaB pathways has been reported in both neurons and microglial cells. The NF-κB pathway targets hundreds of genes, many of which are involved in inflammation, immunity and cancer. p50/p65 (p50/RelA) heterodimers, as the major Rel complex in the NF-κB family, are induced by diverse external physiological stimuli and modulate transcriptional activity in almost all cell types. Both p65 and TDP-43 translocation occur through the classic nuclear transportation system. In this study, we report that TDP-43 overexpression prevents TNF-α induced p65 nuclear translocation in a dose dependent manner, and that this further inhibits p65 transactivation activity. The inhibition by TDP-43 does not occur through preventing IκB degradation but probably by competing for the nuclear transporter-importin α3 (KPNA4). This competition is dependent on the presence of the nuclear localization signal (NLS) in TDP-43. Silencing TDP-43 using a specific siRNA also increased p65 nuclear localization upon TNF-α stimulation, suggesting that endogenous TDP-43 may be a default suppressor of the NF-κB pathway. Our results indicate that TDP-43 may play an important role in regulating the levels of NF-κB activity by controlling the nuclear translocation of p65.
Conflict of interest statement
Figures




Similar articles
-
NF-kappaB p65 regulates nuclear translocation of Ku70 via degradation of heat shock cognate protein 70 in pancreatic acinar AR42J cells.Int J Biochem Cell Biol. 2008;40(10):2065-77. doi: 10.1016/j.biocel.2008.02.015. Epub 2008 Mar 10. Int J Biochem Cell Biol. 2008. PMID: 18378183
-
KPNB1, XPO7 and IPO8 mediate the translocation ofNF-κB/p65 into the nucleus.Traffic. 2013 Nov;14(11):1132-43. doi: 10.1111/tra.12097. Epub 2013 Aug 19. Traffic. 2013. PMID: 23906023
-
p65 controls NF-κB activity by regulating cellular localization of IκBβ.Biochem J. 2011 Mar 1;434(2):253-63. doi: 10.1042/BJ20101220. Biochem J. 2011. PMID: 21158742
-
[Summary and prospect of medicinal plant active substances in regulation of p65 nuclear translocation].Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3286-3293. doi: 10.19540/j.cnki.cjcmm.20170731.003. Zhongguo Zhong Yao Za Zhi. 2017. PMID: 29192437 Review. Chinese.
-
Mechanisms of NF-κB p65 and strategies for therapeutic manipulation.J Inflamm Res. 2018 Oct 30;11:407-419. doi: 10.2147/JIR.S140188. eCollection 2018. J Inflamm Res. 2018. PMID: 30464573 Free PMC article. Review.
Cited by
-
Linear ubiquitination is involved in the pathogenesis of optineurin-associated amyotrophic lateral sclerosis.Nat Commun. 2016 Aug 24;7:12547. doi: 10.1038/ncomms12547. Nat Commun. 2016. PMID: 27552911 Free PMC article.
-
Differential Expression Patterns of TDP-43 in Single Moderate versus Repetitive Mild Traumatic Brain Injury in Mice.Int J Mol Sci. 2021 Nov 11;22(22):12211. doi: 10.3390/ijms222212211. Int J Mol Sci. 2021. PMID: 34830093 Free PMC article.
-
A context-based ABC model for literature-based discovery.PLoS One. 2019 Apr 24;14(4):e0215313. doi: 10.1371/journal.pone.0215313. eCollection 2019. PLoS One. 2019. PMID: 31017923 Free PMC article.
-
Molecular Chaperones' Potential against Defective Proteostasis of Amyotrophic Lateral Sclerosis.Cells. 2023 May 2;12(9):1302. doi: 10.3390/cells12091302. Cells. 2023. PMID: 37174703 Free PMC article. Review.
-
Cytoplasmic poly-GA aggregates impair nuclear import of TDP-43 in C9orf72 ALS/FTLD.Hum Mol Genet. 2017 Feb 15;26(4):790-800. doi: 10.1093/hmg/ddw432. Hum Mol Genet. 2017. PMID: 28040728 Free PMC article.
References
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006. December 22;351(3):602–11. - PubMed
-
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006. October 6;314(5796):130–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

