CD4+ T Cell Tolerance to Tissue-Restricted Self Antigens Is Mediated by Antigen-Specific Regulatory T Cells Rather Than Deletion
- PMID: 26572061
- PMCID: PMC4654997
- DOI: 10.1016/j.immuni.2015.10.011
CD4+ T Cell Tolerance to Tissue-Restricted Self Antigens Is Mediated by Antigen-Specific Regulatory T Cells Rather Than Deletion
Abstract
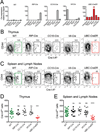
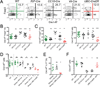
Deletion of self-antigen-specific T cells during thymic development provides protection from autoimmunity. However, it is unclear how efficiently this occurs for tissue-restricted self antigens, or how immune tolerance is maintained for self-antigen-specific T cells that routinely escape deletion. Here we show that endogenous CD4+ T cells with specificity for a set of tissue-restricted self antigens were not deleted at all. For pancreatic self antigen, this resulted in an absence of steady-state tolerance, while for the lung and intestine, tolerance was maintained by the enhanced presence of thymically-derived antigen-specific Foxp3+ regulatory T (Treg) cells. Unlike deletional tolerance, Treg cell-mediated tolerance was broken by successive antigen challenges. These findings reveal that for some tissue-restricted self antigens, tolerance relies entirely on nondeletional mechanisms that are less durable than T cell deletion. This might explain why autoimmunity is often tissue-specific, and it offers a rationale for cancer vaccine strategies targeting tissue-restricted tumor antigens.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Not-So-Negative Selection.Immunity. 2015 Nov 17;43(5):833-5. doi: 10.1016/j.immuni.2015.11.002. Immunity. 2015. PMID: 26588773
References
-
- Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–308. - PubMed
-
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, Boehmer, von H, Bronson R, Dierich A, Benoist C, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. - PubMed
-
- Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

