Development of reverse-transcription loop-mediated isothermal amplification assay for rapid detection and differentiation of dengue virus serotypes 1-4
- PMID: 26572227
- PMCID: PMC4647581
- DOI: 10.1186/s12866-015-0595-1
Development of reverse-transcription loop-mediated isothermal amplification assay for rapid detection and differentiation of dengue virus serotypes 1-4
Abstract
Background: Dengue virus (DENV), the most widely prevalent arbovirus, continues to be a threat to human health in the tropics and subtropics. Early and rapid detection of DENV infection during the acute phase of illness is crucial for proper clinical patient management and preventing the spread of infection. The aim of the current study was to develop a specific, sensitive, and robust reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) assay for detection and differentiation of DENV1-4 serotypes.
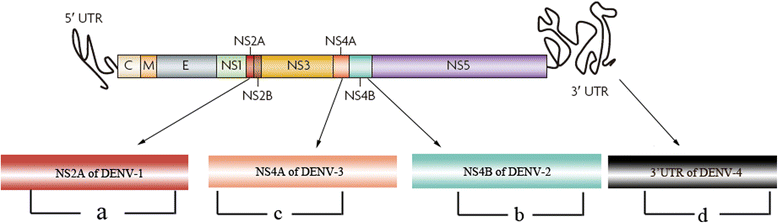
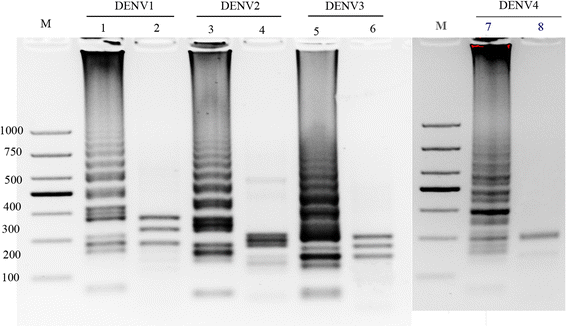
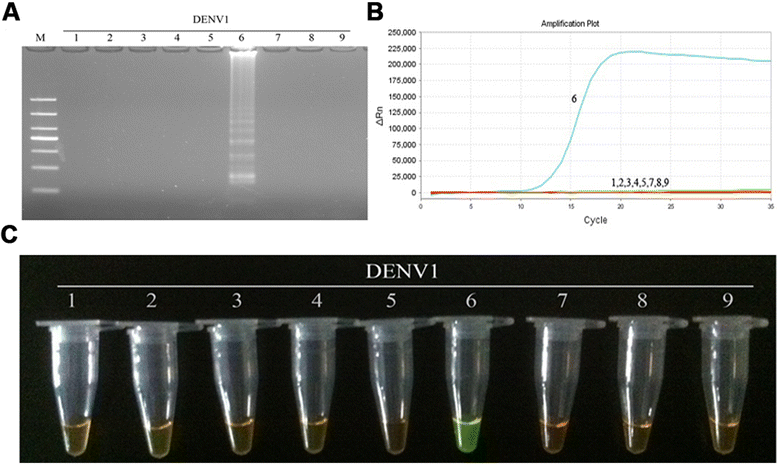
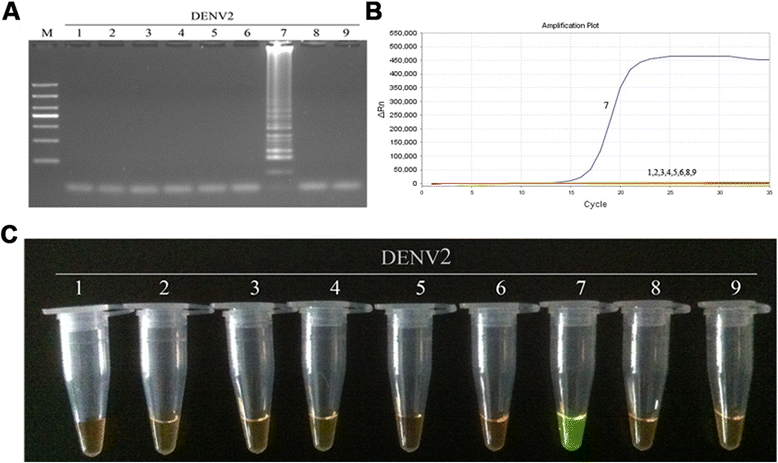
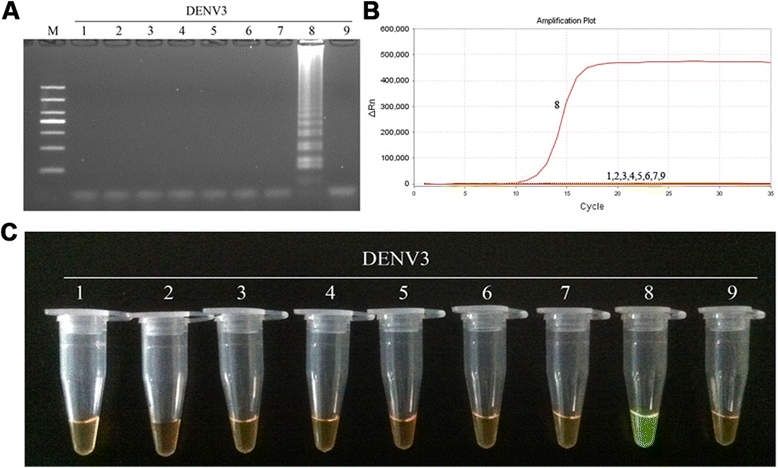
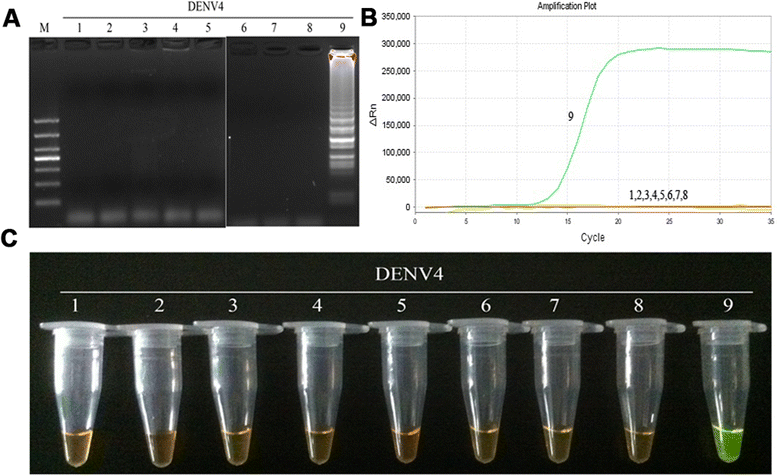
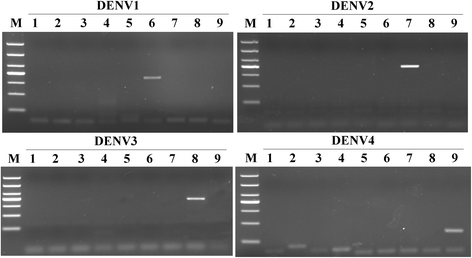
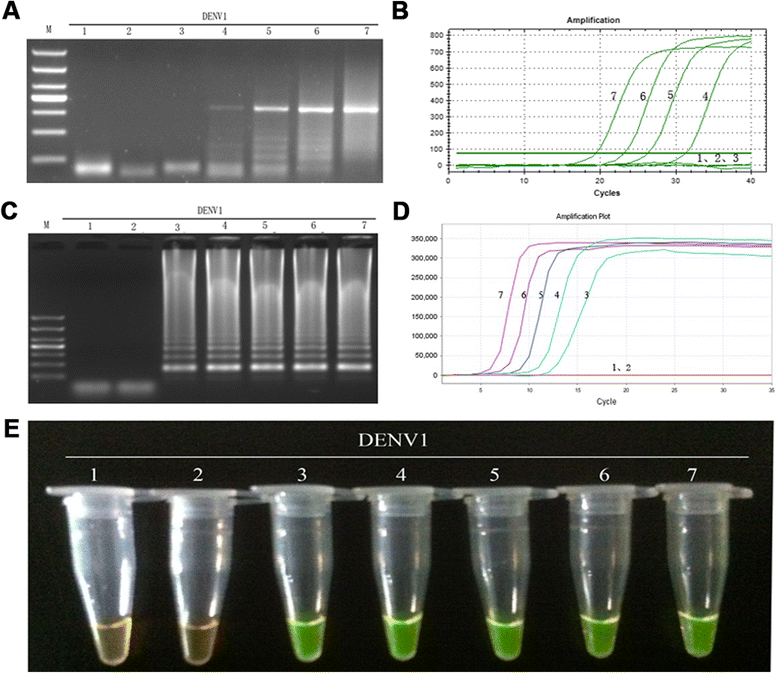
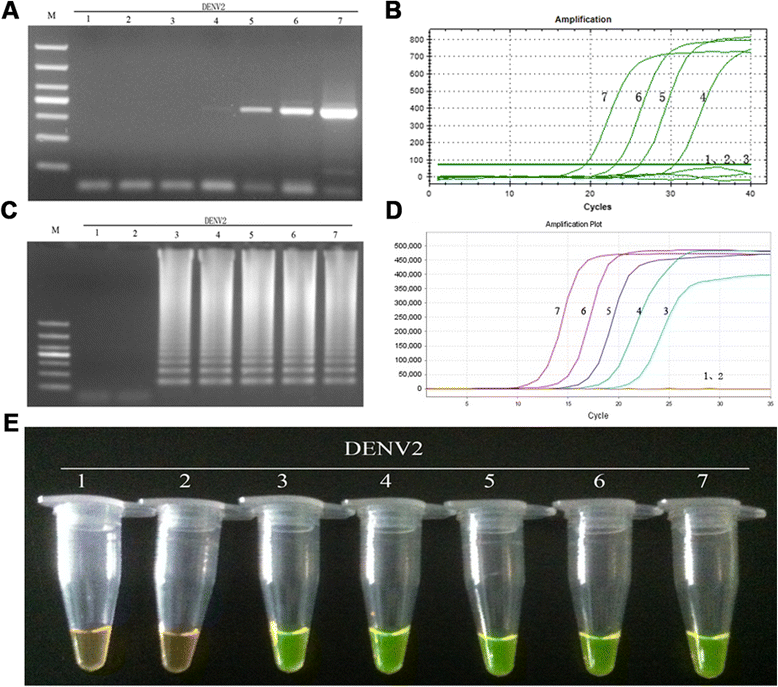
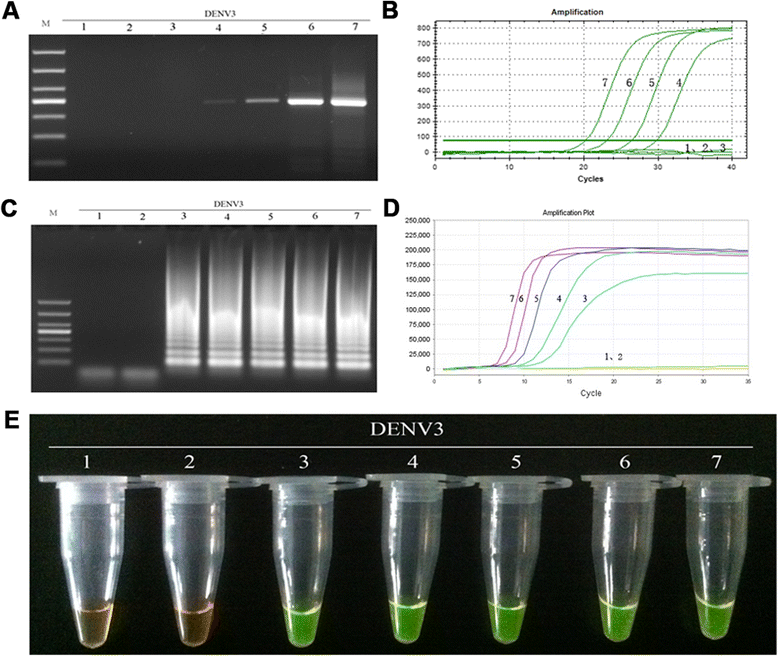
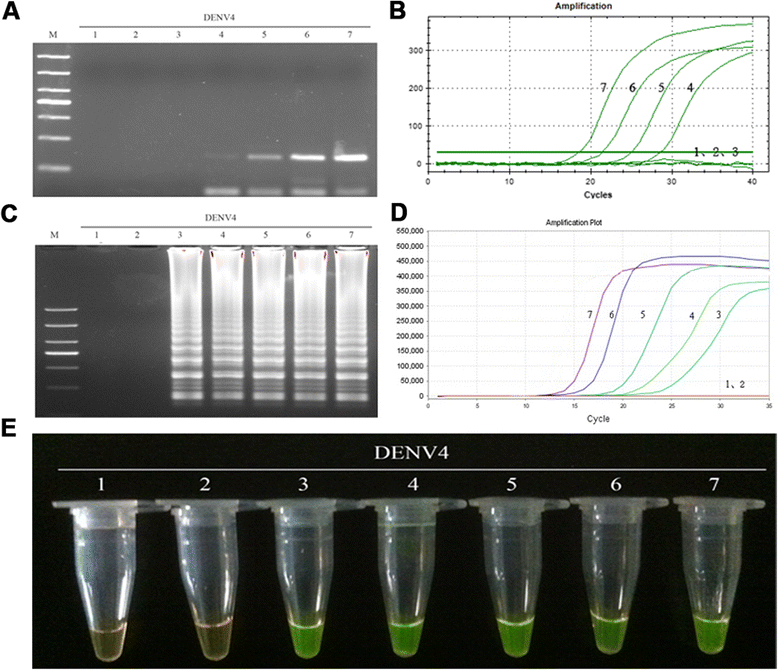
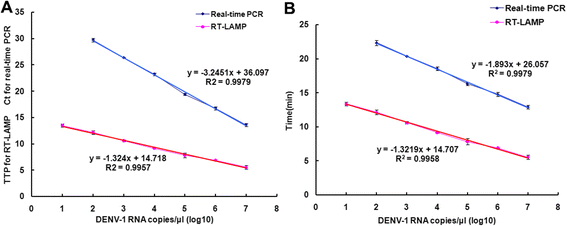
Results: The method detection primers, which were designed to target the different DENV serotypes, were identified by inspection of multiple sequence alignments of the non-structural protein (NS) 2A of DENV1, NS4B of DENV2, NS4A of DENV3 and the 3' untranslated region of the NS protein of DENV4. No cross-reactions of the four serotypes were observed during the tests. The detection limits of the DENV1-4-specific RT-LAMP assays were approximately 10-copy templates per reaction. The RT-LAMP assays were ten-fold more sensitive than RT-PCR or real-time PCR. The diagnostic rate was 100% for clinical strains of DENV, and 98.9% of the DENV-infected patients whose samples were tested were detected by RT-LAMP. Importantly, no false-positives were detected with the new equipment and methodology that was used to avoid aerosol contamination of the samples.
Conclusion: The RT-LAMP method used in our study is specific, sensitive, and suitable for further investigation as a useful alternative to the current methods used for clinical diagnosis of DENV1-4, especially in hospitals and laboratories that lack sophisticated diagnostic systems.
Figures
Similar articles
-
Development and validation of four one-step real-time RT-LAMP assays for specific detection of each dengue virus serotype.PLoS Negl Trop Dis. 2018 May 29;12(5):e0006381. doi: 10.1371/journal.pntd.0006381. eCollection 2018 May. PLoS Negl Trop Dis. 2018. PMID: 29813062 Free PMC article.
-
Rapid detection and differentiation of dengue virus serotypes by NS1 specific reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay in patients presenting to a tertiary care hospital in Hyderabad, India.J Virol Methods. 2015 Jan;211:22-31. doi: 10.1016/j.jviromet.2014.10.005. Epub 2014 Oct 25. J Virol Methods. 2015. PMID: 25455901 Free PMC article.
-
Detection of dengue viruses using reverse transcription-loop-mediated isothermal amplification.BMC Infect Dis. 2013 Aug 21;13:387. doi: 10.1186/1471-2334-13-387. BMC Infect Dis. 2013. PMID: 23964963 Free PMC article.
-
A real-time PCR procedure for detection of dengue virus serotypes 1, 2, and 3, and their quantitation in clinical and laboratory samples.J Virol Methods. 2010 Jan;163(1):1-9. doi: 10.1016/j.jviromet.2009.10.001. Epub 2009 Oct 12. J Virol Methods. 2010. PMID: 19822173 Review.
-
Rapid and sensitive detection of shrimp yellow head virus by loop-mediated isothermal amplification combined with a lateral flow dipstick.J Virol Methods. 2013 Mar;188(1-2):51-6. doi: 10.1016/j.jviromet.2012.11.041. Epub 2012 Dec 7. J Virol Methods. 2013. PMID: 23219929 Review.
Cited by
-
Loop-Mediated Isothermal Amplification (LAMP): The Better Sibling of PCR?Cells. 2021 Jul 29;10(8):1931. doi: 10.3390/cells10081931. Cells. 2021. PMID: 34440699 Free PMC article. Review.
-
Analytical and diagnostic performance characteristics of reverse-transcriptase loop-mediated isothermal amplification assays for dengue virus serotypes 1-4: A scoping review to inform potential use in portable molecular diagnostic devices.PLOS Glob Public Health. 2023 Aug 8;3(8):e0002169. doi: 10.1371/journal.pgph.0002169. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 37552632 Free PMC article.
-
Rapid colorimetric detection of Zika virus from serum and urine specimens by reverse transcription loop-mediated isothermal amplification (RT-LAMP).PLoS One. 2017 Sep 25;12(9):e0185340. doi: 10.1371/journal.pone.0185340. eCollection 2017. PLoS One. 2017. PMID: 28945787 Free PMC article.
-
Quantitative detection of dengue serotypes using a smartphone-connected handheld lab-on-chip platform.Front Bioeng Biotechnol. 2022 Sep 15;10:892853. doi: 10.3389/fbioe.2022.892853. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36185458 Free PMC article.
-
Serotyping dengue virus with isothermal amplification and a portable sequencer.Sci Rep. 2017 Jun 14;7(1):3510. doi: 10.1038/s41598-017-03734-5. Sci Rep. 2017. PMID: 28615658 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

