Major differential gene regulation in Coxiella burnetii between in vivo and in vitro cultivation models
- PMID: 26572556
- PMCID: PMC4647677
- DOI: 10.1186/s12864-015-2143-7
Major differential gene regulation in Coxiella burnetii between in vivo and in vitro cultivation models
Abstract
Background: Coxiella burnetii is the causative agent of the zoonotic disease Q fever. As it is an intracellular pathogen, infection by C. burnetii requires adaptation to its eukaryotic host and intracellular environment. The recently developed cell-free medium also allows the bacteria to propagate without host cells, maintaining its infection potential. The adaptation to different hosts or extracellular environments has been assumed to involve genome-wide modulation of C. burnetii gene expression. However, little is currently known about these adaptation events which are critical for understanding the intracellular survival of C. burnetii.
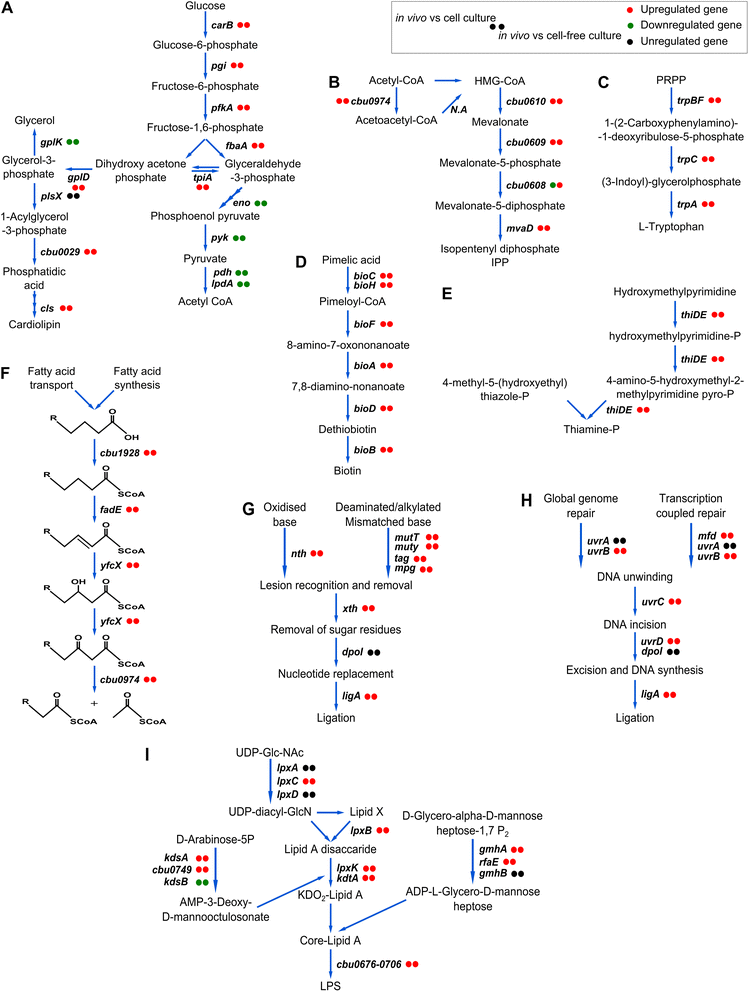
Results: We studied C. burnetii genome-wide transcriptional patterns in vivo (mice spleen) and in cell and cell-free in vitro culture models to examine its metabolic pathways and virulence associated gene expression patterns that are required to colonize and persist in different environments. Within each model, the gene expression profiles of the Dutch C. burnetii outbreak strain (602) and NM reference strains were largely similar. In contrast, modulation of gene-expression was strongly influenced by the cultivation method, indicating adaptation of the bacterium to available components. Genome-wide expression profiles of C. burnetii from in vitro cell culture were more similar to those seen for in vivo conditions, while gene expression profiles of cell-free culture were more distant to in vivo. Under in vivo conditions, significant alterations of genes involved in metabolism and virulence were identified. We observed that C. burnetii under in vivo conditions predominantly uses glucose as a carbon source (mostly for biosynthetic processes) and fatty acids for energy generation. C. burnetii experienced nutrient limitation and anaerobiosis as major stressors, while phosphate limitation was identified as an important signal for intracellular growth inside eukaryotic host cells. Finally, the in vivo environment significantly induced expression of several virulence genes, including those implicated in LPS synthesis, colonization, host component modulation and DNA repair mechanisms.
Conclusion: Our study shows that C. burnetii, with its relative small genome, requires only a subset of core gene functions to survive under in vitro conditions, but requires the induction of full repertoire of genes for successful pathogenesis and thriving in harsh environments in vivo.
Figures
Similar articles
-
Critical Role for Molecular Iron in Coxiella burnetii Replication and Viability.mSphere. 2020 Jul 22;5(4):e00458-20. doi: 10.1128/mSphere.00458-20. mSphere. 2020. PMID: 32699121 Free PMC article.
-
Cell-free propagation of Coxiella burnetii does not affect its relative virulence.PLoS One. 2015 Mar 20;10(3):e0121661. doi: 10.1371/journal.pone.0121661. eCollection 2015. PLoS One. 2015. PMID: 25793981 Free PMC article.
-
Horizontally Acquired Biosynthesis Genes Boost Coxiella burnetii's Physiology.Front Cell Infect Microbiol. 2017 May 10;7:174. doi: 10.3389/fcimb.2017.00174. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28540258 Free PMC article.
-
Role of lipids in Coxiella burnetii infection.Adv Exp Med Biol. 2012;984:199-213. doi: 10.1007/978-94-007-4315-1_10. Adv Exp Med Biol. 2012. PMID: 22711633 Review.
-
Life on the outside: the rescue of Coxiella burnetii from its host cell.Annu Rev Microbiol. 2011;65:111-28. doi: 10.1146/annurev-micro-090110-102927. Annu Rev Microbiol. 2011. PMID: 21639786 Review.
Cited by
-
Coxiella burnetii utilizes both glutamate and glucose during infection with glucose uptake mediated by multiple transporters.Biochem J. 2019 Oct 15;476(19):2851-2867. doi: 10.1042/BCJ20190504. Biochem J. 2019. PMID: 31527117 Free PMC article.
-
Metabolism and physiology of pathogenic bacterial obligate intracellular parasites.Front Cell Infect Microbiol. 2024 Mar 22;14:1284701. doi: 10.3389/fcimb.2024.1284701. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38585652 Free PMC article. Review.
-
Coxiella burnetii replicates in Galleria mellonella hemocytes and transcriptome mapping reveals in vivo regulated genes.Virulence. 2020 Dec;11(1):1268-1278. doi: 10.1080/21505594.2020.1819111. Virulence. 2020. PMID: 32970966 Free PMC article.
-
Transcriptomic Changes of Piscirickettsia salmonis During Intracellular Growth in a Salmon Macrophage-Like Cell Line.Front Cell Infect Microbiol. 2020 Jan 9;9:426. doi: 10.3389/fcimb.2019.00426. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 31998656 Free PMC article.
-
Evaluation of changes to the Rickettsia rickettsii transcriptome during mammalian infection.PLoS One. 2017 Aug 23;12(8):e0182290. doi: 10.1371/journal.pone.0182290. eCollection 2017. PLoS One. 2017. PMID: 28832688 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

