A comparison of fluorescent Ca²⁺ indicators for imaging local Ca²⁺ signals in cultured cells
- PMID: 26572560
- PMCID: PMC4658286
- DOI: 10.1016/j.ceca.2015.10.003
A comparison of fluorescent Ca²⁺ indicators for imaging local Ca²⁺ signals in cultured cells
Abstract
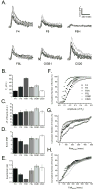
Localized subcellular changes in Ca(2+) serve as important cellular signaling elements, regulating processes as diverse as neuronal excitability and gene expression. Studies of cellular Ca(2+) signaling have been greatly facilitated by the availability of fluorescent Ca(2+) indicators. The respective merits of different indicators to monitor bulk changes in cellular Ca(2+) levels have been widely evaluated, but a comprehensive comparison for their use in detecting and analyzing local, subcellular Ca(2+) signals is lacking. Here, we evaluated several fluorescent Ca(2+) indicators in the context of local Ca(2+) signals (puffs) evoked by inositol 1,4,5-trisphosphate (IP3) in cultured human neuroblastoma SH-SY5Y cells, using high-speed video-microscopy. Altogether, nine synthetic Ca(2+) dyes (Fluo-4, Fluo-8, Fluo-8 high affinity, Fluo-8 low affinity, Oregon Green BAPTA-1, Cal-520, Rhod-4, Asante Calcium Red, and X-Rhod-1) and three genetically-encoded Ca(2+)-indicators (GCaMP6-slow, -medium and -fast variants) were tested; criteria include the magnitude, kinetics, signal-to-noise ratio and detection efficiency of local Ca(2+) puffs. Among these, we conclude that Cal-520 is the optimal indicator for detecting and faithfully tracking local events; that Rhod-4 is the red-emitting indicator of choice; and that none of the GCaMP6 variants are well suited for imaging subcellular Ca(2+) signals.
Keywords: Calcium; Fluorescent indicators; GCaMP6; Genetically encoded calcium indicators; Local calcium signals.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosures
All authors declare that they have no competing financial interests.
Figures





References
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

