Forcible destruction of severely misfolded mammalian glycoproteins by the non-glycoprotein ERAD pathway
- PMID: 26572623
- PMCID: PMC4657166
- DOI: 10.1083/jcb.201504109
Forcible destruction of severely misfolded mammalian glycoproteins by the non-glycoprotein ERAD pathway
Abstract
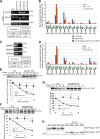
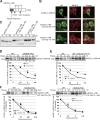
Glycoproteins and non-glycoproteins possessing unfolded/misfolded parts in their luminal regions are cleared from the endoplasmic reticulum (ER) by ER-associated degradation (ERAD)-L with distinct mechanisms. Two-step mannose trimming from Man9GlcNAc2 is crucial in the ERAD-L of glycoproteins. We recently showed that this process is initiated by EDEM2 and completed by EDEM3/EDEM1. Here, we constructed chicken and human cells simultaneously deficient in EDEM1/2/3 and analyzed the fates of four ERAD-L substrates containing three potential N-glycosylation sites. We found that native but unstable or somewhat unfolded glycoproteins, such as ATF6α, ATF6α(C), CD3-δ-ΔTM, and EMC1, were stabilized in EDEM1/2/3 triple knockout cells. In marked contrast, degradation of severely misfolded glycoproteins, such as null Hong Kong (NHK) and deletion or insertion mutants of ATF6α(C), CD3-δ-ΔTM, and EMC1, was delayed only at early chase periods, but they were eventually degraded as in wild-type cells. Thus, higher eukaryotes are able to extract severely misfolded glycoproteins from glycoprotein ERAD and target them to the non-glycoprotein ERAD pathway to maintain the homeostasis of the ER.
© 2015 Ninagawa et al.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

