Tau imaging in neurodegenerative diseases
- PMID: 26572762
- PMCID: PMC4844651
- DOI: 10.1007/s00259-015-3231-2
Tau imaging in neurodegenerative diseases
Abstract
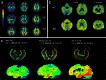
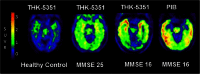
Aggregated tau protein is a major neuropathological substrate central to the pathophysiology of neurodegenerative diseases such as Alzheimer's disease (AD), frontotemporal dementia, progressive supranuclear palsy, corticobasal degeneration and chronic traumatic encephalopathy. In AD, it has been shown that the density of hyperphosphorylated tau tangles correlates closely with neuronal dysfunction and cell death, unlike β-amyloid. Until now, diagnostic and pathologic information about tau deposition has only been available from invasive techniques such as brain biopsy or autopsy. The recent development of selective in-vivo tau PET imaging ligands including [(18)F]THK523, [(18)F]THK5117, [(18)F]THK5105 and [(18)F]THK5351, [(18)F]AV1451(T807) and [(11)C]PBB3 has provided information about the role of tau in the early phases of neurodegenerative diseases, and provided support for diagnosis, prognosis, and imaging biomarkers to track disease progression. Moreover, the spatial and longitudinal relationship of tau distribution compared with β - amyloid and other pathologies in these diseases can be mapped. In this review, we discuss the role of aggregated tau in tauopathies, the challenges posed in developing selective tau ligands as biomarkers, the state of development in tau tracers, and the new clinical information that has been uncovered, as well as the opportunities for improving diagnosis and designing clinical trials in the future.
Keywords: Dementia; Neurodegenerative diseases; Tau imaging.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

