Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease
- PMID: 26572803
- PMCID: PMC4705457
- DOI: 10.1111/cmi.12547
Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease
Abstract
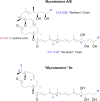
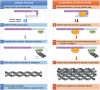
Infection of subcutaneous tissue with Mycobacterium ulcerans can lead to chronic skin ulceration known as Buruli ulcer. The pathogenesis of this neglected tropical disease is dependent on a lipid-like toxin, mycolactone, which diffuses through tissue away from the infecting organisms. Since its identification in 1999, this molecule has been intensely studied to elucidate its cytotoxic and immunosuppressive properties. Two recent major advances identifying the underlying molecular targets for mycolactone have been described. First, it can target scaffolding proteins (such as Wiskott Aldrich Syndrome Protein), which control actin dynamics in adherent cells and therefore lead to detachment and cell death by anoikis. Second, it prevents the co-translational translocation (and therefore production) of many proteins that pass through the endoplasmic reticulum for secretion or placement in cell membranes. These pleiotropic effects underpin the range of cell-specific functional defects in immune and other cells that contact mycolactone during infection. The dose and duration of mycolactone exposure for these different cells explains tissue necrosis and the paucity of immune cells in the ulcers. This review discusses recent advances in the field, revisits older findings in this context and highlights current developments in structure-function studies as well as methodology that make mycolactone a promising diagnostic biomarker.
© 2015 The Authors Cellular Microbiology Published by John Wiley & Sons Ltd.
Figures
References
-
- Adusumilli, S. , Mve‐Obiang, A. , Sparer, T. , Meyers, W. , Hayman, J. , and Small, P.L. (2005) Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo . Cell Microbiol 7: 1295–1304. - PubMed
-
- Boulkroun, S. , Guenin‐Mace, L. , Thoulouze, M.I. , Monot, M. , Merckx, A. , Langsley, G. , et al. (2010) Mycolactone suppresses T cell responsiveness by altering both early signaling and posttranslational events. J Immunol 184: 1436–1444. - PubMed
-
- Chany, A.C. , Casarotto, V. , Schmitt, M. , Tarnus, C. , Guenin‐Mace, L. , Demangel, C. , et al. (2011) A diverted total synthesis of mycolactone analogues: an insight into Buruli ulcer toxins. Chemistry 17: 14413–14419. - PubMed
-
- Chany, A.C. , Veyron‐Churlet, R. , Tresse, C. , Mayau, V. , Casarotto, V. , Le Chevalier, F. , et al. (2014) Synthetic variants of mycolactone bind and activate Wiskott‐Aldrich syndrome proteins. J Med Chem 57: 7382–7395. - PubMed
-
- Connor, D.H. , and Lunn, H.F. (1965) Mycobacterium ulcerans infection (with comments on pathogenesis). Int J Lepr 33(Suppl): 698–709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

