Auranofin is a potent suppressor of osteosarcoma metastasis
- PMID: 26573231
- PMCID: PMC4808036
- DOI: 10.18632/oncotarget.5704
Auranofin is a potent suppressor of osteosarcoma metastasis
Abstract
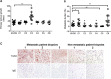
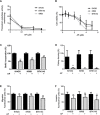
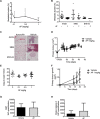
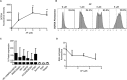
Osteosarcoma (OS) accounts for 56% of malignant bone cancers in children and adolescents. Patients with localized disease rarely develop metastasis; however, pulmonary metastasis occurs in approximately 50% of patients and leads to a 5-year survival rate of only 10-20%. Therefore, identifying the genes and pathways involved in metastasis, as new therapeutic targets, is crucial to improve long-term survival of OS patients. Novel markers that define metastatic OS were identified using comparative transcriptomic analyses of two highly metastatic (C1 and C6) and two poorly metastatic clonal variants (C4 and C5) isolated from the metastatic OS cell line, KHOS. Using this approach, we determined that the metastatic phenotype correlated with overexpression of thioredoxin reductase 2 (TXNRD2) or vascular endothelial growth factor (VEGF). Validation in patient biopsies confirmed TXNRD2 and VEGF targets were highly expressed in 29-42% of metastatic OS patient biopsies, with no detectable expression in non-malignant bone or samples from OS patients with localised disease. Auranofin (AF) was used to selectively target and inhibit thioredoxin reductase (TrxR). At low doses, AF was able to inhibit TrxR activity without a significant effect on cell viability whereas at higher doses, AF could induce ROS-dependent apoptosis. AF treatment, in vivo, significantly reduced the development of pulmonary metastasis and we provide evidence that this effect may be due to an AF-dependent increase in cellular ROS. Thus, TXNRD2 may represent a novel druggable target that could be deployed to reduce the development of fatal pulmonary metastases in patients with OS.
Keywords: auranofin; metastasis; osteosarcoma; oxidative stress; thioredoxin reductase.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures

References
-
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer treatment and research. 2009;152:3–13. - PubMed
-
- Chou AJ, Geller DS, Gorlick R. Therapy for osteosarcoma: where do we go from here? Paediatric drugs. 2008;10:315–327. - PubMed
-
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012;366:883–892. - PMC - PubMed
-
- Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nature reviews. Cancer. 2012;12:323–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

