Interpreting small treatment differences from quality of life data in cancer trials: an alternative measure of treatment benefit and effect size for the EORTC-QLQ-C30
- PMID: 26573600
- PMCID: PMC4647515
- DOI: 10.1186/s12955-015-0374-6
Interpreting small treatment differences from quality of life data in cancer trials: an alternative measure of treatment benefit and effect size for the EORTC-QLQ-C30
Abstract
Background: The EORTC-QLQ-C30 is a widely used health related quality of life (HRQoL) questionnaire in lung cancer patients. Small HRQoL treatment effects are often reported as mean differences (MDs) between treatments, which are rarely justified or understood by patients and clinicians. An alternative approach using odds ratios (OR) for reporting effects is proposed. This may offer advantages including facilitating alignment between patient and clinician understanding of HRQoL effects.
Methods: Data from six CRUK sponsored randomized controlled lung cancer trials (2 small cell and 4 in non-small cell, in 2909 patients) were used to HRQoL effects. Results from Beta-Binomial (BB) standard mixed effects were compared. Preferences for ORs vs MDs were determined and Time to Deterioration (TD) was also compared.
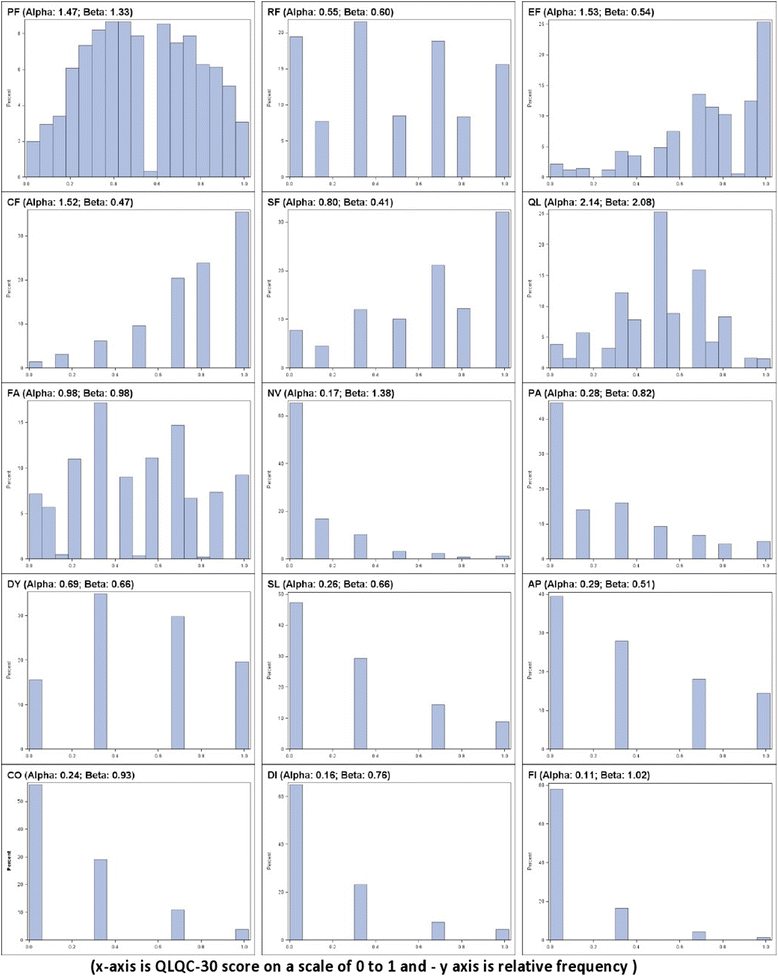
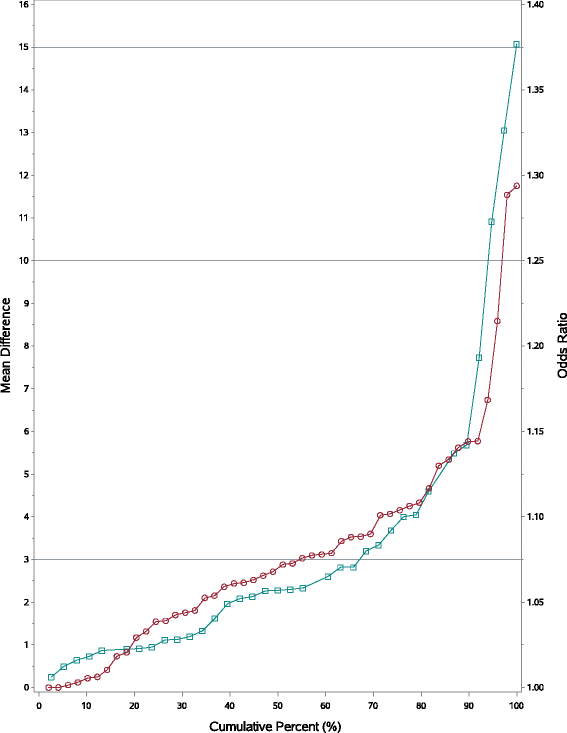
Results: HRQoL effects using ORs offered coherent interpretations: MDs >0 resulted in ORs >1 and vice versa; effect sizes were classified as 'Trivial' if the OR was between 1 ± 0.05 (i.e. 0.95 to 1.05); 'Small': for 1 ± 0.1; 'Medium': 1 ± 0.2 and 'Large': OR <0.8 or >1.20. Small HRQoL effects on the MD scale may translate to important treatment differences on the OR scale: for example, a worsening in symptoms (MD) by 2.6 points (p = 0.1314) would be a 17 % deterioration (p < 0.0001) with an OR. Hence important differences may be missed with MD; conversely, small ORs are unlikely to yield large MDs because methods based on OR model skewed data well. Initial evidence also suggests oncologists prefer ORs over MDs since interpretation is similar to hazard ratios.
Conclusion: Reporting HRQoL benefits as MDs can be misleading. Estimates of HRQoL treatment effects in terms of ORs are preferred over MDs. Future analysis of QLQ-C30 and other HRQoL measures should consider reporting HRQoL treatment effects as ORs.
Figures
Similar articles
-
Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials.Support Care Cancer. 2011 Nov;19(11):1753-60. doi: 10.1007/s00520-010-1016-5. Epub 2010 Oct 1. Support Care Cancer. 2011. PMID: 20886240
-
Interpreting European Organisation for Research and Treatment for Cancer Quality of life Questionnaire core 30 scores as minimally importantly different for patients with malignant melanoma.Eur J Cancer. 2018 Nov;104:169-181. doi: 10.1016/j.ejca.2018.09.005. Epub 2018 Oct 22. Eur J Cancer. 2018. PMID: 30359910
-
Reliability of an e-PRO Tool of EORTC QLQ-C30 for Measurement of Health-Related Quality of Life in Patients With Breast Cancer: Prospective Randomized Trial.J Med Internet Res. 2017 Sep 14;19(9):e322. doi: 10.2196/jmir.8210. J Med Internet Res. 2017. PMID: 28912116 Free PMC article. Clinical Trial.
-
Methodology of health-related quality of life analysis in phase III advanced non-small-cell lung cancer clinical trials: a critical review.BMC Cancer. 2016 Feb 18;16:122. doi: 10.1186/s12885-016-2152-1. BMC Cancer. 2016. PMID: 26892541 Free PMC article. Review.
-
Methodological issues in assessing health-related quality of life of colorectal cancer patients in randomised controlled trials.Eur J Cancer. 2004 Jan;40(2):187-97. doi: 10.1016/j.ejca.2003.10.012. Eur J Cancer. 2004. PMID: 14728932 Review.
Cited by
-
Effectiveness of the HuCare Quality Improvement Strategy on health-related quality of life in patients with cancer: study protocol of a stepped-wedge cluster randomised controlled trial (HuCare2 study).BMJ Open. 2017 Oct 6;7(10):e016347. doi: 10.1136/bmjopen-2017-016347. BMJ Open. 2017. PMID: 28988170 Free PMC article. Clinical Trial.
-
Comparison of statistical methods for the analysis of patient-reported outcomes (PROs), particularly the Short-Form 36 (SF-36), in randomised controlled trials (RCTs) using standardised effect size (SES): an empirical analysis.Health Qual Life Outcomes. 2025 Apr 29;23(1):45. doi: 10.1186/s12955-025-02373-z. Health Qual Life Outcomes. 2025. PMID: 40301969 Free PMC article.
-
Effectiveness of a Psychosocial Care Quality Improvement Strategy to Address Quality of Life in Patients With Cancer: The HuCare2 Stepped-Wedge Cluster Randomized Trial.JAMA Netw Open. 2021 Oct 1;4(10):e2128667. doi: 10.1001/jamanetworkopen.2021.28667. JAMA Netw Open. 2021. PMID: 34648011 Free PMC article. Clinical Trial.
-
Role of radiofrequency ablation and cement injection for pain control in patients with spinal metastasis.BMC Musculoskelet Disord. 2021 Oct 29;22(1):912. doi: 10.1186/s12891-021-04799-0. BMC Musculoskelet Disord. 2021. PMID: 34715849 Free PMC article.
-
Cognitive bias modification for facial interpretation: a randomized controlled trial of transfer to self-report and cognitive measures in a healthy sample.R Soc Open Sci. 2017 Dec 13;4(12):170681. doi: 10.1098/rsos.170681. eCollection 2017 Dec. R Soc Open Sci. 2017. PMID: 29308221 Free PMC article.
References
-
- Lemonnier I, Guillemin F, Arveux P, Clément-Duchêne C, Velten M, Woronoff-Lemsi MC, et al. Quality of life after the initial treatments of non-small cell lung cancer: a persistent predictor for patients' survival. Health Qual Life Outcomes. 2014;12:73. doi: 10.1186/1477-7525-12-73. - DOI - PMC - PubMed
-
- Cancer Research UK. (2014a). Lung cancer Key Facts. Available: http://www.cancerresearchuk.org/cancer-info/cancerstats/keyfacts/lung-ca.... Last accessed 10th Oct 2015.
-
- Brazier, J.E., Rowen, D. NICE DSU Technical Support Document 11: Alternatives to EQ-5D for generating health state utility values; 2011. Available from http://www.nicedsu.org.uk. - PubMed
-
- Khan. I; Design & Analysis of Clinical Trials For Cost-effectiveness & Reimbursement; 315 Pages Chapman & Hall; 2015 (in press)
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

