A general mechanism for drug promiscuity: Studies with amiodarone and other antiarrhythmics
- PMID: 26573624
- PMCID: PMC4664825
- DOI: 10.1085/jgp.201511470
A general mechanism for drug promiscuity: Studies with amiodarone and other antiarrhythmics
Abstract
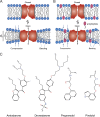
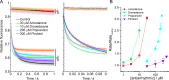
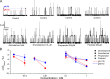
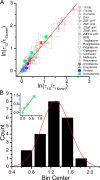
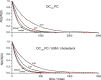
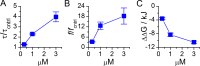
Amiodarone is a widely prescribed antiarrhythmic drug used to treat the most prevalent type of arrhythmia, atrial fibrillation (AF). At therapeutic concentrations, amiodarone alters the function of many diverse membrane proteins, which results in complex therapeutic and toxicity profiles. Other antiarrhythmics, such as dronedarone, similarly alter the function of multiple membrane proteins, suggesting that a multipronged mechanism may be beneficial for treating AF, but raising questions about how these antiarrhythmics regulate a diverse range of membrane proteins at similar concentrations. One possible mechanism is that these molecules regulate membrane protein function by altering the common environment provided by the host lipid bilayer. We took advantage of the gramicidin (gA) channels' sensitivity to changes in bilayer properties to determine whether commonly used antiarrhythmics--amiodarone, dronedarone, propranolol, and pindolol, whose pharmacological modes of action range from multi-target to specific--perturb lipid bilayer properties at therapeutic concentrations. Using a gA-based fluorescence assay, we found that amiodarone and dronedarone are potent bilayer modifiers at therapeutic concentrations; propranolol alters bilayer properties only at supratherapeutic concentration, and pindolol has little effect. Using single-channel electrophysiology, we found that amiodarone and dronedarone, but not propranolol or pindolol, increase bilayer elasticity. The overlap between therapeutic and bilayer-altering concentrations, which is observed also using plasma membrane-like lipid mixtures, underscores the need to explore the role of the bilayer in therapeutic as well as toxic effects of antiarrhythmic agents.
© 2015 Rusinova et al.
Figures
Similar articles
-
Dronedarone or amiodarone for rhythm control for atrial fibrillation: implications from the DIONYSOS study.Expert Opin Pharmacother. 2010 Dec;11(17):2775-8. doi: 10.1517/14656566.2010.517196. Expert Opin Pharmacother. 2010. PMID: 21050033
-
Dronedarone: a promising alternative for the management of atrial fibrillation.Cardiovasc Drugs Ther. 2009 Oct;23(5):385-93. doi: 10.1007/s10557-009-6189-0. Cardiovasc Drugs Ther. 2009. PMID: 19669399 Review.
-
Augmenting maintenance of sinus rhythm in the control of atrial fibrillation by antiarrhythmic drug combinations.J Cardiovasc Pharmacol Ther. 2010 Dec;15(4 Suppl):31S-5S. doi: 10.1177/1074248410377617. J Cardiovasc Pharmacol Ther. 2010. PMID: 21098417
-
Dronedarone: Basic Pharmacology and Clinical Use.Card Electrophysiol Clin. 2016 Jun;8(2):453-65. doi: 10.1016/j.ccep.2016.02.008. Epub 2016 Mar 19. Card Electrophysiol Clin. 2016. PMID: 27261834 Review.
-
Dronedarone: an overview.Ann Med. 2012 Feb;44(1):60-72. doi: 10.3109/07853890.2011.594808. Epub 2011 Jul 11. Ann Med. 2012. PMID: 21745093 Review.
Cited by
-
Mechanisms underlying drug-mediated regulation of membrane protein function.Proc Natl Acad Sci U S A. 2021 Nov 16;118(46):e2113229118. doi: 10.1073/pnas.2113229118. Proc Natl Acad Sci U S A. 2021. PMID: 34753824 Free PMC article.
-
Multiple neurosteroid and cholesterol binding sites in voltage-dependent anion channel-1 determined by photo-affinity labeling.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Oct;1864(10):1269-1279. doi: 10.1016/j.bbalip.2019.06.004. Epub 2019 Jun 5. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 31176038 Free PMC article.
-
Cannabidiol increases gramicidin current in human embryonic kidney cells: An observational study.PLoS One. 2022 Aug 1;17(8):e0271801. doi: 10.1371/journal.pone.0271801. eCollection 2022. PLoS One. 2022. PMID: 35913948 Free PMC article.
-
Synthetic Analogues of the Snail Toxin 6-Bromo-2-mercaptotryptamine Dimer (BrMT) Reveal That Lipid Bilayer Perturbation Does Not Underlie Its Modulation of Voltage-Gated Potassium Channels.Biochemistry. 2018 May 8;57(18):2733-2743. doi: 10.1021/acs.biochem.8b00292. Epub 2018 Apr 17. Biochemistry. 2018. PMID: 29616558 Free PMC article.
-
Dimeric Tubulin Modifies Mechanical Properties of Lipid Bilayer, as Probed Using Gramicidin A Channel.Int J Mol Sci. 2024 Feb 12;25(4):2204. doi: 10.3390/ijms25042204. Int J Mol Sci. 2024. PMID: 38396879 Free PMC article.
References
-
- Anavekar S.-N., Louis W.J., Morgan T.O., Doyle A.E., and Johnston C.I.. 1975. The relationship of plasma levels of pindolol in hypertensive patients to effects on blood pressure, plasma renin and plasma noradrenaline levels. Clin. Exp. Pharmacol. Physiol. 2:203–212. 10.1111/j.1440-1681.1975.tb03026.x - DOI - PubMed
-
- Andersen O.S., Sawyer D.B., and Koeppe R.E. II. 1992. Modulation of channel function by the host bilayer. Biomembrane Structure and Function. Easwaran K.R.K. and Gaber B., Adenine Press, Schenectady, NY: 227–244.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

