Nrf2-Mediated Cardiac Maladaptive Remodeling and Dysfunction in a Setting of Autophagy Insufficiency
- PMID: 26573705
- PMCID: PMC4837959
- DOI: 10.1161/HYPERTENSIONAHA.115.06062
Nrf2-Mediated Cardiac Maladaptive Remodeling and Dysfunction in a Setting of Autophagy Insufficiency
Abstract
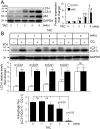
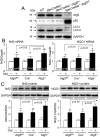
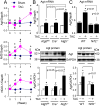
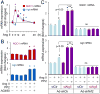
Nuclear factor erythroid-2-related factor 2 (Nrf2) appears to exert either a protective or detrimental effect on the heart; however, the underlying mechanism remains poorly understood. Herein, we uncovered a novel mechanism for turning off the Nrf2-mediated cardioprotection and switching on Nrf2-mediated cardiac dysfunction. In a murine model of pressure overload-induced cardiac remodeling and dysfunction via transverse aortic arch constriction, knockout of Nrf2 enhanced myocardial necrosis and death rate during an initial stage of cardiac adaptation when myocardial autophagy function is intact. However, knockout of Nrf2 turned out to be cardioprotective throughout the later stage of cardiac maladaptive remodeling when myocardial autophagy function became insufficient. Transverse aortic arch constriction -induced activation of Nrf2 was dramatically enhanced in the heart with impaired autophagy, which is induced by cardiomyocyte-specific knockout of autophagy-related gene (Atg)5. Notably, Nrf2 activation coincided with the upregulation of angiotensinogen (Agt) only in the autophagy-impaired heart after transverse aortic arch constriction. Agt5 and Nrf2 gene loss-of-function approaches in combination with Jak2 and Fyn kinase inhibitors revealed that suppression of autophagy inactivated Jak2 and Fyn and nuclear translocation of Fyn, while enhancing nuclear translocation of Nrf2 and Nrf2-driven Agt expression in cardiomyocytes. Taken together, these results indicate that the pathophysiological consequences of Nrf2 activation are closely linked with the functional integrity of myocardial autophagy during cardiac remodeling. When autophagy is intact, Nrf2 is required for cardiac adaptive responses; however, autophagy impairment most likely turns off Fyn-operated Nrf2 nuclear export thus activating Nrf2-driven Agt transcription, which exacerbates cardiac maladaptation leading to dysfunction.
Keywords: NF-E2-related factor 2; angiotensinogen; autophagy; myocardial infarction; ventricular remodeling.
© 2015 American Heart Association, Inc.
Figures




Similar articles
-
Deubiquitinating enzyme CYLD mediates pressure overload-induced cardiac maladaptive remodeling and dysfunction via downregulating Nrf2.J Mol Cell Cardiol. 2015 Jul;84:143-53. doi: 10.1016/j.yjmcc.2015.04.012. Epub 2015 Apr 30. J Mol Cell Cardiol. 2015. PMID: 25935309
-
Nrf2 enhances myocardial clearance of toxic ubiquitinated proteins.J Mol Cell Cardiol. 2014 Jul;72:305-15. doi: 10.1016/j.yjmcc.2014.04.006. Epub 2014 Apr 18. J Mol Cell Cardiol. 2014. PMID: 24747945 Free PMC article.
-
Nrf2 protects against maladaptive cardiac responses to hemodynamic stress.Arterioscler Thromb Vasc Biol. 2009 Nov;29(11):1843-50. doi: 10.1161/ATVBAHA.109.189480. Epub 2009 Jul 10. Arterioscler Thromb Vasc Biol. 2009. PMID: 19592468
-
The role of Nrf2-mediated pathway in cardiac remodeling and heart failure.Oxid Med Cell Longev. 2014;2014:260429. doi: 10.1155/2014/260429. Epub 2014 Jul 1. Oxid Med Cell Longev. 2014. PMID: 25101151 Free PMC article. Review.
-
NRF2 in Cardiovascular Diseases: a Ray of Hope!J Cardiovasc Transl Res. 2021 Jun;14(3):573-586. doi: 10.1007/s12265-020-10083-8. Epub 2020 Nov 25. J Cardiovasc Transl Res. 2021. PMID: 33241490 Review.
Cited by
-
Insights into the Molecular Mechanisms of NRF2 in Kidney Injury and Diseases.Int J Mol Sci. 2023 Mar 23;24(7):6053. doi: 10.3390/ijms24076053. Int J Mol Sci. 2023. PMID: 37047024 Free PMC article. Review.
-
Oxidative Stress in Arterial Hypertension (HTN): The Nuclear Factor Erythroid Factor 2-Related Factor 2 (Nrf2) Pathway, Implications and Future Perspectives.Pharmaceutics. 2022 Feb 27;14(3):534. doi: 10.3390/pharmaceutics14030534. Pharmaceutics. 2022. PMID: 35335911 Free PMC article. Review.
-
Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3ß/Fyn pathway.Redox Biol. 2018 May;15:405-417. doi: 10.1016/j.redox.2017.12.016. Epub 2018 Jan 2. Redox Biol. 2018. PMID: 29353218 Free PMC article.
-
The P21-Activated Kinase 1 and 2 As Potential Therapeutic Targets for the Management of Cardiovascular Disease.Int J Drug Discov Pharm. 2022 Dec 21:5. doi: 10.53941/ijddp.v1i1.179. Online ahead of print. Int J Drug Discov Pharm. 2022. PMID: 39899001 Free PMC article.
-
Autophagy Controls Nrf2-Mediated Dichotomy in Pressure Overloaded Hearts.Front Physiol. 2021 May 13;12:673145. doi: 10.3389/fphys.2021.673145. eCollection 2021. Front Physiol. 2021. PMID: 34054582 Free PMC article.
References
-
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the keap1-nrf2-are pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. - PubMed
-
- Li J, Ichikawa T, Janicki JS, Cui T. Targeting the nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets. 2009;13:785–794. - PubMed
-
- Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of nrf2, keap1: A historical overview. Antioxidants & redox signaling. 2010;13:1665–1678. - PubMed
-
- Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, Cui T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–1850. - PubMed
-
- Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, Sporn MB, Gabrielson KL, Champion HC, Tuder RM, Kensler TW, Biswal S. Targeting nrf2 with the triterpenoid cddo-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA. 2009;106:250–255. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

