Obesity Contributes to Ovarian Cancer Metastatic Success through Increased Lipogenesis, Enhanced Vascularity, and Decreased Infiltration of M1 Macrophages
- PMID: 26573796
- PMCID: PMC4668203
- DOI: 10.1158/0008-5472.CAN-15-0706
Obesity Contributes to Ovarian Cancer Metastatic Success through Increased Lipogenesis, Enhanced Vascularity, and Decreased Infiltration of M1 Macrophages
Abstract
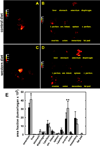
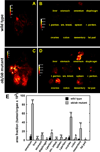
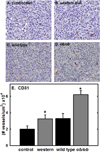
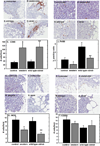
Epithelial ovarian cancer (EOC) is the leading cause of death from gynecologic malignancy, with high mortality attributable to widespread intraperitoneal metastases. Recent meta-analyses report an association between obesity, ovarian cancer incidence, and ovarian cancer survival, but the effect of obesity on metastasis has not been evaluated. The objective of this study was to use an integrative approach combining in vitro, ex vivo, and in vivo studies to test the hypothesis that obesity contributes to ovarian cancer metastatic success. Initial in vitro studies using three-dimensional mesomimetic cultures showed enhanced cell-cell adhesion to the lipid-loaded mesothelium. Furthermore, in an ex vivo colonization assay, ovarian cancer cells exhibited increased adhesion to mesothelial explants excised from mice modeling diet-induced obesity (DIO), in which they were fed a "Western" diet. Examination of mesothelial ultrastructure revealed a substantial increase in the density of microvilli in DIO mice. Moreover, enhanced intraperitoneal tumor burden was observed in overweight or obese animals in three distinct in vivo models. Further histologic analyses suggested that alterations in lipid regulatory factors, enhanced vascularity, and decreased M1/M2 macrophage ratios may account for the enhanced tumorigenicity. Together, these findings show that obesity potently affects ovarian cancer metastatic success, which likely contributes to the negative correlation between obesity and ovarian cancer survival.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures



References
-
- U.S. Cancer Statistics Working Group. Incidence and Mortality Web Based Report. Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2014. United States Cancer Statisitics: 1999–2011.
-
- Jemal A, Bray F, Ferlay J. Global cancer statistics. 2011;61:69–90. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. CA Cancer J. Clin. 2013;63:11–30. - PubMed
-
- American Cancer Society. Cancer Facts and figures. Atlanta: 2013.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

