Defective Store-Operated Calcium Entry Causes Partial Nephrogenic Diabetes Insipidus
- PMID: 26574044
- PMCID: PMC4926963
- DOI: 10.1681/ASN.2014121200
Defective Store-Operated Calcium Entry Causes Partial Nephrogenic Diabetes Insipidus
Abstract
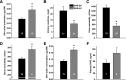
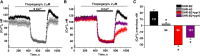
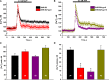
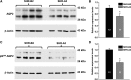
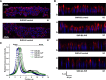
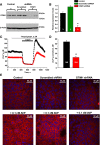
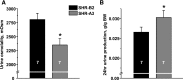
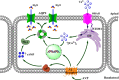
Store-operated calcium entry (SOCE) is the mechanism by which extracellular signals elicit prolonged intracellular calcium elevation to drive changes in fundamental cellular processes. Here, we investigated the role of SOCE in the regulation of renal water reabsorption, using the inbred rat strain SHR-A3 as an animal model with disrupted SOCE. We found that SHR-A3, but not SHR-B2, have a novel truncating mutation in the gene encoding stromal interaction molecule 1 (STIM1), the endoplasmic reticulum calcium (Ca(2+)) sensor that triggers SOCE. Balance studies revealed increased urine volume, hypertonic plasma, polydipsia, and impaired urinary concentrating ability accompanied by elevated circulating arginine vasopressin (AVP) levels in SHR-A3 compared with SHR-B2. Isolated, split-open collecting ducts (CD) from SHR-A3 displayed decreased basal intracellular Ca(2+) levels and a major defect in SOCE. Consequently, AVP failed to induce the sustained intracellular Ca(2+) mobilization that requires SOCE in CD cells from SHR-A3. This effect decreased the abundance of aquaporin 2 and enhanced its intracellular retention, suggesting impaired sensitivity of the CD to AVP in SHR-A3. Stim1 knockdown in cultured mpkCCDc14 cells reduced SOCE and basal intracellular Ca(2+) levels and prevented AVP-induced translocation of aquaporin 2, further suggesting the effects in SHR-A3 result from the expression of truncated STIM1. Overall, these results identify a novel mechanism of nephrogenic diabetes insipidus and uncover a role of SOCE in renal water handling.
Keywords: calcium; collecting ducts; diabetes insipidus; vasopressin; water transport.
Copyright © 2016 by the American Society of Nephrology.
Figures


Comment in
-
Water, Water Everywhere: A New Cause and a New Treatment for Nephrogenic Diabetes Insipidus.J Am Soc Nephrol. 2016 Jul;27(7):1872-4. doi: 10.1681/ASN.2015111223. Epub 2015 Dec 28. J Am Soc Nephrol. 2016. PMID: 26712528 Free PMC article. No abstract available.
References
-
- Moeller HB, Fenton RA: Cell biology of vasopressin-regulated aquaporin-2 trafficking. Pflugers Arch 464: 133–144, 2012 - PubMed
-
- Robertson GL: Physiology of ADH secretion. Kidney Int Suppl 21: S20–S26, 1987 - PubMed
-
- Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA: Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

