Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity
- PMID: 26574055
- PMCID: PMC4648063
- DOI: 10.1038/srep16865
Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity
Abstract
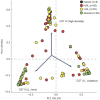
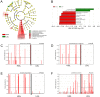
Persistent infection with oncogenic Human Papillomavirus (HPV) is necessary for cervical carcinogenesis. Although evidence suggests that the vaginal microbiome plays a functional role in the persistence or regression of HPV infections, this has yet to be described in women with cervical intra-epithelial neoplasia (CIN). We hypothesised that increasing microbiome diversity is associated with increasing CIN severity. llumina MiSeq sequencing of 16S rRNA gene amplicons was used to characterise the vaginal microbiota of women with low-grade squamous intra-epithelial lesions (LSIL; n = 52), high-grade (HSIL; n = 92), invasive cervical cancer (ICC; n = 5) and healthy controls (n = 20). Hierarchical clustering analysis revealed an increased prevalence of microbiomes characterised by high-diversity and low levels of Lactobacillus spp. (community state type-CST IV) with increasing disease severity, irrespective of HPV status (Normal = 2/20,10%; LSIL = 11/52,21%; HSIL = 25/92,27%; ICC = 2/5,40%). Increasing disease severity was associated with decreasing relative abundance of Lactobacillus spp. The vaginal microbiome in HSIL was characterised by higher levels of Sneathia sanguinegens (P < 0.01), Anaerococcus tetradius (P < 0.05) and Peptostreptococcus anaerobius (P < 0.05) and lower levels of Lactobacillus jensenii (P < 0.01) compared to LSIL. Our results suggest advancing CIN disease severity is associated with increasing vaginal microbiota diversity and may be involved in regulating viral persistence and disease progression.
Figures


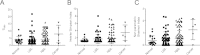
References
-
- Munoz N. Human papillomavirus and cancer: the epidemiological evidence. J. Clin. Virol. 19, 1–5 (2000). - PubMed
-
- Myers E. R., McCrory D. C., Nanda K., Bastian L. & Matchar D. B. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am. J. Epidemiol. 151, 1158–1171 (2000). - PubMed
-
- Richardson H. et al.. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol. Biomarkers Prev. 12, 485–490 (2003). - PubMed
-
- Boskey E. R., Cone R. A., Whaley K. J. & Moench T. R. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 16, 1809–1813 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

