Heightening Energetic Stress Selectively Targets LKB1-Deficient Non-Small Cell Lung Cancers
- PMID: 26574479
- PMCID: PMC4654699
- DOI: 10.1158/0008-5472.CAN-15-0797
Heightening Energetic Stress Selectively Targets LKB1-Deficient Non-Small Cell Lung Cancers
Abstract
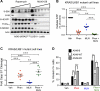
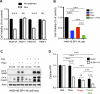
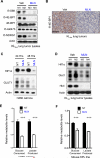
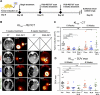
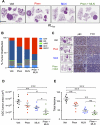
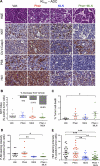
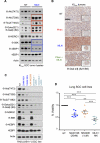
Inactivation of the LKB1 tumor suppressor is a frequent event in non-small cell lung carcinoma (NSCLC) leading to the activation of mTOR complex 1 (mTORC1) and sensitivity to the metabolic stress inducer phenformin. In this study, we explored the combinatorial use of phenformin with the mTOR catalytic kinase inhibitor MLN0128 as a treatment strategy for NSCLC bearing comutations in the LKB1 and KRAS genes. NSCLC is a genetically and pathologically heterogeneous disease, giving rise to lung tumors of varying histologies that include adenocarcinomas and squamous cell carcinomas (SCC). We demonstrate that phenformin in combination with MLN0128 induced a significant therapeutic response in KRAS/LKB1-mutant human cell lines and genetically engineered mouse models of NSCLC that develop both adenocarcinomas and SCCs. Specifically, we found that KRAS/LKB1-mutant lung adenocarcinomas responded strongly to phenformin + MLN0128 treatment, but the response of SCCs to single or combined treatment with MLN0128 was more attenuated due to acquired resistance to mTOR inhibition through modulation of the AKT-GSK signaling axis. Combinatorial use of the mTOR inhibitor and AKT inhibitor MK2206 robustly inhibited the growth and viability of squamous lung tumors, thus providing an effective strategy to overcome resistance. Taken together, our findings define new personalized therapeutic strategies that may be rapidly translated into clinical use for the treatment of KRAS/LKB1-mutant adenocarcinomas and squamous cell tumors.
©2015 American Association for Cancer Research.
Figures
References
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. - PubMed
-
- Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous Cell Carcinoma of the Lung: Molecular Subtypes and Therapeutic Opportunities. Clin Cancer Res. 2012;18(9):2443–51. - PubMed
-
- Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62(13):3659–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

