The Non-structural Protein of Crimean-Congo Hemorrhagic Fever Virus Disrupts the Mitochondrial Membrane Potential and Induces Apoptosis
- PMID: 26574543
- PMCID: PMC4705379
- DOI: 10.1074/jbc.M115.667436
The Non-structural Protein of Crimean-Congo Hemorrhagic Fever Virus Disrupts the Mitochondrial Membrane Potential and Induces Apoptosis
Abstract
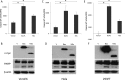
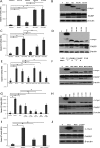
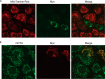
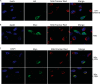
Viruses have developed distinct strategies to overcome the host defense system. Regulation of apoptosis in response to viral infection is important for virus survival and dissemination. Like other viruses, Crimean-Congo hemorrhagic fever virus (CCHFV) is known to regulate apoptosis. This study, for the first time, suggests that the non-structural protein NSs of CCHFV, a member of the genus Nairovirus, induces apoptosis. In this report, we demonstrated the expression of CCHFV NSs, which contains 150 amino acid residues, in CCHFV-infected cells. CCHFV NSs undergoes active degradation during infection. We further demonstrated that ectopic expression of CCHFV NSs induces apoptosis, as reflected by caspase-3/7 activity and cleaved poly(ADP-ribose) polymerase, in different cell lines that support CCHFV replication. Using specific inhibitors, we showed that CCHFV NSs induces apoptosis via both intrinsic and extrinsic pathways. The minimal active region of the CCHFV NSs protein was determined to be 93-140 amino acid residues. Using alanine scanning, we demonstrated that Leu-127 and Leu-135 are the key residues for NSs-induced apoptosis. Interestingly, CCHFV NSs co-localizes in mitochondria and also disrupts the mitochondrial membrane potential. We also demonstrated that Leu-127 and Leu-135 are important residues for disruption of the mitochondrial membrane potential by NSs. Therefore, these results indicate that the C terminus of CCHFV NSs triggers mitochondrial membrane permeabilization, leading to activation of caspases, which, ultimately, leads to apoptosis. Given that multiple factors contribute to apoptosis during CCHFV infection, further studies are needed to define the involvement of CCHFV NSs in regulating apoptosis in infected cells.
Keywords: Crimean-Congo hemorrhagic fever virus; apoptosis; caspase; mitochondria; mitochondrial membrane potential; non-structural protein; proteasome.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Whitehouse C. A. (2004) Crimean-Congo hemorrhagic fever. Antiviral Res. 64, 145–160 - PubMed
-
- Keshtkar-Jahromi M., Kuhn J. H., Christova I., Bradfute S. B., Jahrling P. B., and Bavari S. (2011) Crimean-Congo hemorrhagic fever: current and future prospects of vaccines and therapies. Antiviral Res. 90, 85–92 - PubMed
-
- Bente D. A., Forrester N. L., Watts D. M., McAuley A. J., Whitehouse C. A., and Bray M. (2013) Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 100, 159–189 - PubMed
-
- Elliott R. M. (1990) Molecular biology of the Bunyaviridae. J. Gen. Virol. 71, 501–522 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

