Propranolol Targets Hemangioma Stem Cells via cAMP and Mitogen-Activated Protein Kinase Regulation
- PMID: 26574555
- PMCID: PMC4704871
- DOI: 10.5966/sctm.2015-0076
Propranolol Targets Hemangioma Stem Cells via cAMP and Mitogen-Activated Protein Kinase Regulation
Abstract
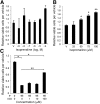
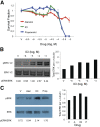
Infantile hemangiomas (IHs) are the most common vascular tumor and arise from a hemangioma stem cell (HemSC). Propranolol has proved efficacious for problematic IHs. Propranolol is a nonselective β-adrenergic receptor (βAR) antagonist that can lower cAMP levels and activate the mitogen-activated protein kinase (MAPK) pathway downstream of βARs. We found that HemSCs express β1AR and β2AR in proliferating IHs and determined the role of these βARs and the downstream pathways in mediating propranolol's effects. In isolated HemSCs, propranolol suppressed cAMP levels and activated extracellular signal-regulated kinase (ERK)1/2 in a dose-dependent fashion. Propranolol, used at doses of <10(-4) M, reduced cAMP levels and decreased HemSC proliferation and viability. Propranolol at ≥10(-5) M reduced cAMP levels and activated ERK1/2, and this correlated with HemSC apoptosis and cytotoxicity at ≥10(-4) M. Stimulation with a βAR agonist, isoprenaline, promoted HemSC proliferation and rescued the antiproliferative effects of propranolol, suggesting that propranolol inhibits βAR signaling in HemSCs. Treatment with a cAMP analog or a MAPK inhibitor partially rescued the HemSC cell viability suppressed by propranolol. A selective β2AR antagonist mirrored propranolol's effects on HemSCs in a dose-dependent fashion, and a selective β1AR antagonist had no effect, supporting a role for β2AR signaling in IH pathobiology. In a mouse model of IH, propranolol reduced the vessel caliber and blood flow assessed by ultrasound Doppler and increased activation of ERK1/2 in IH cells. We have thus demonstrated that propranolol acts on HemSCs in IH to suppress proliferation and promote apoptosis in a dose-dependent fashion via β2AR perturbation, resulting in reduced cAMP and MAPK activation.
Significance: The present study investigated the action of propranolol in infantile hemangiomas (IHs). IHs are the most common vascular tumor in children and have been proposed to arise from a hemangioma stem cell (HemSC). Propranolol, a nonselective β-adrenergic receptor (βAR) antagonist, has proven efficacy; however, understanding of its mechanism of action on HemSCs is limited. The presented data demonstrate that propranolol, via βAR perturbation, dose dependently suppresses cAMP levels and activated extracellular signal-regulated kinase 1/2. Furthermore, propranolol acts via perturbation of β2AR, and not β1AR, although both receptors are expressed in HemSCs. These results provide important insight into propranolol's action in IHs and can be used to guide the development of more targeted therapy.
Keywords: Cell death; Cell proliferation; Hemangioma; Mitogen-activated protein kinase; Propranolol; Stem cell; cAMP; β-Adrenergic receptor.
©AlphaMed Press.
Figures





References
-
- Mulliken JB, Fishman SJ, Burrows PE. Vascular anomalies. Curr Probl Surg. 2000;37:517–584. - PubMed
-
- Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: Current knowledge, future directions. Proceedings of a research workshop on infantile hemangiomas, April 7–9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol. 2005;22:383–406. - PubMed
-
- Kwon EK, Seefeldt M, Drolet BA. Infantile hemangiomas: An update. Am J Clin Dermatol. 2013;14:111–123. - PubMed
-
- Chamlin SL, Haggstrom AN, Drolet BA, et al. Multicenter prospective study of ulcerated hemangiomas. J Pediatr. 2007;151:684–689. - PubMed
-
- Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: Clinical characteristics predicting complications and treatment. Pediatrics. 2006;118:882–887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

