Destabilizing RET in targeted treatment of thyroid cancers
- PMID: 26574568
- PMCID: PMC4674629
- DOI: 10.1530/EC-15-0098
Destabilizing RET in targeted treatment of thyroid cancers
Abstract
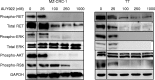
Metastatic differentiated thyroid cancers (DTC) are resistant to traditional chemotherapy. Kinase inhibitors have shown promise in patients with progressive DTC, but dose-limiting toxicity is commonplace. HSP90 regulates protein degradation of several growth-mediating kinases such as RET, and we hypothesized that HSP90 inhibitor (AUY922) could inhibit RET-mediated medullary thyroid cancer (MTC) as well as papillary thyroid cancer (PTC) cell growth and also radioactive iodine uptake by PTC cells. Studies utilized MTC cell lines TT (C634W) and MZ-CRC-1 (M918T) and the PTC cell line TPC-1 (RET/PTC1). Cell viability was assessed with MTS assays and apoptosis by flow cytometry. Signaling target expression was determined by western blot and radioiodine uptake measured with a gamma counter. Prolonged treatment of both MTC cell lines with AUY922 simultaneously inhibited both MAPK and mTOR pathways and significantly induced apoptosis (58.7 and 78.7% reduction in MZ-CRC-1 and TT live cells respectively, following 1 μM AUY922; P<0.02). Similarly in the PTC cell line, growth and signaling targets were inhibited, and also a 2.84-fold increase in radioiodine uptake was observed following AUY922 administration (P=0.015). AUY922 demonstrates in vitro activity against MTC and PTC cell lines. We observed a potent dose-dependent increase in apoptosis in MTC cell lines following drug administration confirming its anti-tumorigenic effects. Western blots confirm inhibition of pro-survival proteins including AKT suggesting this as the mechanism of cell death. In a functional study, we observed an increase in radioiodine uptake in the PTC cell line following AUY922 treatment. We believe HSP90 inhibition could be a viable alternative for treatment of RET-driven chemo-resistant thyroid cancers.
Keywords: AUY922; HSP90; MAPK; RET; mTOR; thyroid cancer.
© 2015 The authors.
Figures





References
-
- Park JW, Yeh MW, Wong MG, Lobo M, Hyun WC, Duh QY, Clark OH. The heat shock protein 90-binding geldanamycin inhibits cancer cell proliferation, down-regulates oncoproteins, and inhibits epidermal growth factor-induced invasion in thyroid cancer cell lines. Journal of Clinical Endocrinology and Metabolism. 2003;88:3346–3353. doi: 10.1210/jc.2002-020340. - DOI - PubMed
-
- Kamal A, Burrows FJ. Hsp90 inhibitors as selective anticancer drugs. Discovery Medicine. 2004;4:277–280. doi: 10.1097/01.cad.0000136876.11928.be. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

