Blood-Brain Barrier Disruption Is an Early Event That May Persist for Many Years After Traumatic Brain Injury in Humans
- PMID: 26574669
- PMCID: PMC8744142
- DOI: 10.1097/NEN.0000000000000261
Blood-Brain Barrier Disruption Is an Early Event That May Persist for Many Years After Traumatic Brain Injury in Humans
Abstract
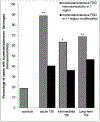
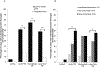
Traumatic brain injury (TBI) is a risk factor for dementia. Mixed neurodegenerative pathologies have been described in late survivors of TBI, but the mechanisms driving post-TBI neurodegeneration remain elusive. Increasingly, blood-brain barrier (BBB) disruption has been recognized in a range of neurologic disorders including dementias, but little is known of the consequences of TBI on the BBB. Autopsy cases of single moderate or severe TBI from the Glasgow TBI Archive (n = 70) were selected to include a range from acute (10 hours-13 days) to long-term (1-47 years) survival, together with age-matched uninjured controls (n = 21). Multiple brain regions were examined using immunohistochemistry for the BBB integrity markers fibrinogen and immunoglobulin G. After TBI, 40% of patients dying in the acute phase and 47% of those surviving a year or more from injury showed multifocal, abnormal, perivascular, and parenchymal fibrinogen and immunoglobulin G immunostaining localized to the gray matter, with preferential distribution toward the crests of gyri and deep neocortical layers. In contrast, when present, controls showed only limited localized immunostaining. These preliminary data demonstrate evidence of widespread BBB disruption in a proportion of TBI patients emerging in the acute phase and, intriguingly, persisting in a high proportion of late survivors.
Figures



References
-
- Coronado VG, McGuire LC, Sarmiento K, Bell J, Lionbarger MR, Jones CD, Geller AI, Khoury N, Xu L. Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009. J Safety Res 2012:43;299–307. - PubMed
-
- Molgaard CA, Stanford EP, Morton DJ, Ryden LA, Schubert KR, Golbeck AL. Epidemiology of head trauma and neurocognitive impairment in a multi-ethnic population. Neuroepidemiology 1990:9;233–42. - PubMed
-
- Mortimer JA, French LR, Hutton JT, Schuman LM. Head injury as a risk factor for Alzheimer’s disease. Neurology 1985:35;264–7. - PubMed
-
- Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Rocca WA, Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 1991:20 Suppl 2;S28–35. - PubMed
-
- Graves AB, White E, Koepsell TD, Reifler BV, van Belle G, Larson EB, Raskind M. The association between head trauma and Alzheimer’s disease. Am J Epidemiol1990:131;491–501.1990:131;491–501. - PubMed

