Protein composition of 6K2-induced membrane structures formed during Potato virus A infection
- PMID: 26574906
- PMCID: PMC6638329
- DOI: 10.1111/mpp.12341
Protein composition of 6K2-induced membrane structures formed during Potato virus A infection
Abstract
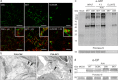
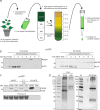
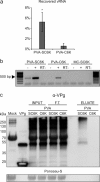
The definition of the precise molecular composition of membranous replication compartments is a key to understanding the mechanisms of virus multiplication. Here, we set out to investigate the protein composition of the potyviral replication complexes. We purified the potyviral 6K2 protein-induced membranous structures from Potato virus A (PVA)-infected Nicotiana benthamiana plants. For this purpose, the 6K2 protein, which is the main inducer of potyviral membrane rearrangements, was expressed in fusion with an N-terminal Twin-Strep-tag and Cerulean fluorescent protein (SC6K) from the infectious PVA cDNA. A non-tagged Cerulean-6K2 (C6K) virus and the SC6K protein alone in the absence of infection were used as controls. A purification scheme exploiting discontinuous sucrose gradient centrifugation followed by Strep-tag-based affinity chromatography was developed. Both (+)- and (-)-strand PVA RNA and viral protein VPg were co-purified specifically with the affinity tagged PVA-SC6K. The purified samples, which contained individual vesicles and membrane clusters, were subjected to mass spectrometry analysis. Data analysis revealed that many of the detected viral and host proteins were either significantly enriched or fully specifically present in PVA-SC6K samples when compared with the controls. Eight of eleven potyviral proteins were identified with high confidence from the purified membrane structures formed during PVA infection. Ribosomal proteins were identified from the 6K2-induced membranes only in the presence of a replicating virus, reinforcing the tight coupling between replication and translation. A substantial number of proteins associating with chloroplasts and several host proteins previously linked with potyvirus replication complexes were co-purified with PVA-derived SC6K, supporting the conclusion that the host proteins identified in this study may have relevance in PVA replication.
Keywords: 6K2 protein; Potato virus A; potyvirus; proteome; viral replication complex.
© 2015 BSPP AND JOHN WILEY & SONS LTD.
Figures


Similar articles
-
Tobacco vein banding mosaic virus 6K2 Protein Hijacks NbPsbO1 for Virus Replication.Sci Rep. 2017 Feb 23;7:43455. doi: 10.1038/srep43455. Sci Rep. 2017. PMID: 28230184 Free PMC article.
-
Interaction network of tobacco etch potyvirus NIa protein with the host proteome during infection.BMC Genomics. 2016 Feb 1;17:87. doi: 10.1186/s12864-016-2394-y. BMC Genomics. 2016. PMID: 26830344 Free PMC article.
-
Helper component proteinase of the genus Potyvirus is an interaction partner of translation initiation factors eIF(iso)4E and eIF4E and contains a 4E binding motif.J Virol. 2011 Jul;85(13):6784-94. doi: 10.1128/JVI.00485-11. Epub 2011 Apr 27. J Virol. 2011. PMID: 21525344 Free PMC article.
-
The Molecular Maze of Potyviral and Host Protein Interactions.Annu Rev Virol. 2024 Sep;11(1):147-170. doi: 10.1146/annurev-virology-100422-034124. Epub 2024 Aug 30. Annu Rev Virol. 2024. PMID: 38848589 Review.
-
The mystery remains: How do potyviruses move within and between cells?Mol Plant Pathol. 2023 Dec;24(12):1560-1574. doi: 10.1111/mpp.13383. Epub 2023 Aug 12. Mol Plant Pathol. 2023. PMID: 37571979 Free PMC article. Review.
Cited by
-
A Functional Link between RNA Replication and Virion Assembly in the Potyvirus Plum Pox Virus.J Virol. 2018 Apr 13;92(9):e02179-17. doi: 10.1128/JVI.02179-17. Print 2018 May 1. J Virol. 2018. PMID: 29444942 Free PMC article.
-
AtHVA22a, a plant-specific homologue of Reep/DP1/Yop1 family proteins is involved in turnip mosaic virus propagation.Mol Plant Pathol. 2024 May;25(5):e13466. doi: 10.1111/mpp.13466. Mol Plant Pathol. 2024. PMID: 38767756 Free PMC article.
-
Small hydrophobic viral proteins involved in intercellular movement of diverse plant virus genomes.AIMS Microbiol. 2020 Sep 21;6(3):305-329. doi: 10.3934/microbiol.2020019. eCollection 2020. AIMS Microbiol. 2020. PMID: 33134746 Free PMC article. Review.
-
Interplay of HCPro and CP in the Regulation of Potato Virus A RNA Expression and Encapsidation.Viruses. 2022 Jun 7;14(6):1233. doi: 10.3390/v14061233. Viruses. 2022. PMID: 35746704 Free PMC article.
-
Imaging Techniques to Study Plant Virus Replication and Vertical Transmission.Viruses. 2021 Feb 25;13(3):358. doi: 10.3390/v13030358. Viruses. 2021. PMID: 33668729 Free PMC article. Review.
References
-
- Aniento, F. , Matsuoka, K. and Robinson, D.G. (2006) ER‐to‐Golgi transport: the COPII‐pathway In: The Plant Endoplasmic Reticulum (Robinson D.G., ed.), pp. 99–124. Plant Cell Monographs Berlin, Heidelberg: Springer.
-
- Anindya, R. , Chittori, S. and Savithri, H.S. (2005) Tyrosine 66 of Pepper vein banding virus genome‐linked protein is uridylylated by RNA‐dependent RNA polymerase. Virology, 336, 154–162. - PubMed
-
- den Boon, J.A. and Ahlquist, P. (2010) Organelle‐like membrane compartmentalization of positive‐strand RNA virus replication factories. Annu. Rev. Microbiol. 64, 241–256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

