Increased DUX4 expression during muscle differentiation correlates with decreased SMCHD1 protein levels at D4Z4
- PMID: 26575099
- PMCID: PMC4844215
- DOI: 10.1080/15592294.2015.1113798
Increased DUX4 expression during muscle differentiation correlates with decreased SMCHD1 protein levels at D4Z4
Erratum in
-
Corrigendum. Increased DUX4 expression during muscle differentiation correlates with decreased SMCHD1 protein levels at D4Z4.Epigenetics. 2016;11(2):175. doi: 10.1080/15592294.2016.1161273. Epigenetics. 2016. PMID: 27058812 Free PMC article. No abstract available.
Abstract
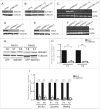
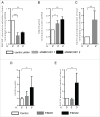
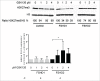
Facioscapulohumeral muscular dystrophy is caused by incomplete epigenetic repression of the transcription factor DUX4 in skeletal muscle. A copy of DUX4 is located within each unit of the D4Z4 macrosatellite repeat array and its derepression in somatic cells is caused by either repeat array contraction (FSHD1) or by mutations in the chromatin repressor SMCHD1 (FSHD2). While DUX4 expression has thus far only been detected in FSHD muscle and muscle cell cultures, and increases with in vitro myogenic differentiation, the D4Z4 chromatin structure has only been studied in proliferating myoblasts or non-myogenic cells. We here show that SMCHD1 protein levels at D4Z4 decline during muscle cell differentiation and correlate with DUX4 derepression. In FSHD2, but not FSHD1, the loss of SMCHD1 repressor activity is partially compensated by increased Polycomb Repressive Complex 2 (PRC2)-mediated H3K27 trimethylation at D4Z4, a situation that can be mimicked by SMCHD1 knockdown in control myotubes. In contrast, moderate overexpression of SMCHD1 results in DUX4 silencing in FSHD1 and FSHD2 myotubes demonstrating that DUX4 derepression in FSHD is reversible. Together, we show that in FSHD1 and FSHD2 the decline in SMCHD1 protein levels during muscle cell differentiation renders skeletal muscle sensitive to DUX4.
Keywords: D4Z4; DUX4; FSHD; Polycomb Repressive Complex 2; SMCHD1; myogenesis; transcriptional regulation.
Figures

References
-
- Tawil R, van der Maarel SM, Tapscott SJ. Facioscapulohumeral dystrophy: the path to consensus on pathophysiology. Skelet Muscle 2014; 4:12; PMID:24940479; http://dx.doi.org/10.1186/2044-5040-4-12 - DOI - PMC - PubMed
-
- Deenen JC, Arnts H, van der Maarel SM, Padberg GW, Verschuuren JJ, Bakker E, Weinreich SS, Verbeek AL, van Engelen BG. Population-based incidence and prevalence of facioscapulohumeral dystrophy. Neurology 2014; 83:1056–9; PMID:25122204; http://dx.doi.org/10.1212/WNL.0000000000000797 - DOI - PMC - PubMed
-
- Gabriels J, Beckers MC, Ding H, De Vriese A, Plaisance S, van der Maarel SM, Padberg GW, Frants RR, Hewitt JE, Collen D, et al. . Nucleotide sequence of the partially deleted D4Z4 locus in a patient with FSHD identifies a putative gene within each 3.3 kb element. Gene 1999; 236:25–32; PMID:10433963; http://dx.doi.org/10.1016/S0378-1119(99)00267-X - DOI - PubMed
-
- Wijmenga C, Frants RR, Brouwer OF, Moerer P, Weber JL, Padberg GW. Location of facioscapulohumeral muscular dystrophy gene on chromosome 4. Lancet 1990; 336:651–3; PMID:1975852; http://dx.doi.org/10.1016/0140-6736(90)92148-B - DOI - PubMed
-
- Wijmenga C, Hewitt JE, Sandkuijl LA, Clark LN, Wright TJ, Dauwerse HG, Gruter AM, Hofker MH, Moerer P, Williamson R, et al. . Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat Genet 1992; 2:26–30; PMID:1363881; http://dx.doi.org/10.1038/ng0992-26 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
