Evolutionary origins of hepatitis A virus in small mammals
- PMID: 26575627
- PMCID: PMC4679062
- DOI: 10.1073/pnas.1516992112
Evolutionary origins of hepatitis A virus in small mammals
Abstract
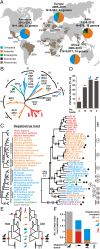
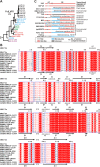
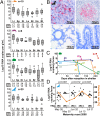
Hepatitis A virus (HAV) is an ancient and ubiquitous human pathogen recovered previously only from primates. The sole species of the genus Hepatovirus, existing in both enveloped and nonenveloped forms, and with a capsid structure intermediate between that of insect viruses and mammalian picornaviruses, HAV is enigmatic in its origins. We conducted a targeted search for hepatoviruses in 15,987 specimens collected from 209 small mammal species globally and discovered highly diversified viruses in bats, rodents, hedgehogs, and shrews, which by pairwise sequence distance comprise 13 novel Hepatovirus species. Near-complete genomes from nine of these species show conservation of unique hepatovirus features, including predicted internal ribosome entry site structure, a truncated VP4 capsid protein lacking N-terminal myristoylation, a carboxyl-terminal pX extension of VP1, VP2 late domains involved in membrane envelopment, and a cis-acting replication element within the 3D(pol) sequence. Antibodies in some bat sera immunoprecipitated and neutralized human HAV, suggesting conservation of critical antigenic determinants. Limited phylogenetic cosegregation among hepatoviruses and their hosts and recombination patterns are indicative of major hepatovirus host shifts in the past. Ancestral state reconstructions suggest a Hepatovirus origin in small insectivorous mammals and a rodent origin of human HAV. Patterns of infection in small mammals mimicked those of human HAV in hepatotropism, fecal shedding, acute nature, and extinction of the virus in a closed host population. The evolutionary conservation of hepatovirus structure and pathogenesis provide novel insight into the origins of HAV and highlight the utility of analyzing animal reservoirs for risk assessment of emerging viruses.
Keywords: hepatitis A virus; pathogenesis; small mammals; viral evolution; zoonosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Comment in
-
Human hepatitis A virus is united with a host of relations.Proc Natl Acad Sci U S A. 2015 Dec 8;112(49):15010-1. doi: 10.1073/pnas.1520121112. Epub 2015 Nov 18. Proc Natl Acad Sci U S A. 2015. PMID: 26582796 Free PMC article. No abstract available.
References
-
- Luis AD, et al. 2013. A comparison of bats and rodents as reservoirs of zoonotic viruses: Are bats special? Proc R Soc Biol Sci 280(1756):20122753.
-
- Leroy EM, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438(7068):575–576. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

