Palladium-Catalyzed Benzylic C-H Arylation of Azaarylmethylamines
- PMID: 26576005
- PMCID: PMC4880366
- DOI: 10.1021/acs.orglett.5b02898
Palladium-Catalyzed Benzylic C-H Arylation of Azaarylmethylamines
Abstract
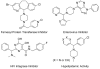
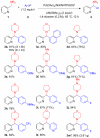
A direct C-H functionalization approach to produce aryl(azaaryl)methylamines from azaarylmethylamines without directing groups is described. Under conditions where the azaarylmethylamines' C-H is reversibly deprotonated, a Pd(OAc)(2)/NIXANTPHOS-based catalyst couples the resulting carbanions with various aryl halides to provide aryl(azaaryl)methylamines. This umpolung strategy directly provides tertiary amines without protecting or activating groups.
Figures




Similar articles
-
Ruthenium(II)-catalyzed sp3 C-H bond arylation of benzylic amines using aryl halides.Org Lett. 2012 Jul 20;14(14):3792-5. doi: 10.1021/ol301680v. Epub 2012 Jul 12. Org Lett. 2012. PMID: 22789033
-
α-Arylation of Saturated Azacycles and N-Methylamines via Palladium(II)-Catalyzed C(sp(3))-H Coupling.J Am Chem Soc. 2015 Sep 23;137(37):11876-9. doi: 10.1021/jacs.5b06740. Epub 2015 Sep 10. J Am Chem Soc. 2015. PMID: 26322957 Free PMC article.
-
Palladium-catalyzed benzylic arylation of 2-methyl azaarenes.Org Lett. 2010 Dec 3;12(23):5359-61. doi: 10.1021/ol102276e. Epub 2010 Nov 2. Org Lett. 2010. PMID: 21043493
-
Palladium(0)-Catalyzed Benzylic C(sp3)-H Functionalization for the Concise Synthesis of Heterocycles and Its Applications.Chem Pharm Bull (Tokyo). 2017;65(5):409-425. doi: 10.1248/cpb.c16-00969. Chem Pharm Bull (Tokyo). 2017. PMID: 28458363 Review.
-
Palladium-Catalyzed Direct Synthesis and Insecticidal Activity of Arylmatrine Derivatives.J Nat Prod. 2022 Aug 26;85(8):2026-2034. doi: 10.1021/acs.jnatprod.2c00417. Epub 2022 Aug 3. J Nat Prod. 2022. PMID: 35920623 Review.
Cited by
-
Regio- and chemoselective Csp3-H arylation of benzylamines by single electron transfer/hydrogen atom transfer synergistic catalysis.Chem Sci. 2018 Sep 12;9(44):8453-8460. doi: 10.1039/c8sc02965b. eCollection 2018 Nov 28. Chem Sci. 2018. PMID: 30542595 Free PMC article.
-
Palladium-Catalyzed Triarylation of sp3 C-H Bonds in Heteroarylmethanes: Synthesis of Triaryl(heteroaryl)methanes.Adv Synth Catal. 2018 Apr 3;360(7):1493-1498. doi: 10.1002/adsc.201701347. Epub 2018 Feb 3. Adv Synth Catal. 2018. PMID: 30093851 Free PMC article.
-
Palladium-catalyzed benzylic C(sp3)-H carbonylative arylation of azaarylmethyl amines with aryl bromides.Chem Sci. 2021 Jul 8;12(32):10862-10870. doi: 10.1039/d1sc02078a. eCollection 2021 Aug 18. Chem Sci. 2021. PMID: 34476065 Free PMC article.
-
Palladium-Catalyzed Selective α-Alkenylation of Pyridylmethyl Ethers with Vinyl Bromides.Org Lett. 2016 May 20;18(10):2371-4. doi: 10.1021/acs.orglett.6b00815. Epub 2016 May 10. Org Lett. 2016. PMID: 27160421 Free PMC article.
-
Palladium-Catalyzed Benzylic Arylation of Pyridylmethyl Silyl Ethers: One-Pot Synthesis of Aryl(pyridyl)methanols.Org Lett. 2016 Apr 1;18(7):1590-3. doi: 10.1021/acs.orglett.6b00450. Epub 2016 Mar 23. Org Lett. 2016. PMID: 27004592 Free PMC article.
References
-
- Henry GD. Tetrahedron. 2004;60:6043.
- Michael JP. Nat. Prod. Rep. 2005;22:627. - PubMed
- Laird T. Org. Process Res. Dev. 2006;10:851.
- Joule JA, Mills K. Heterocyclic Chemistry. 5th ed John Wiley & Sons; Chichester, U.K.: 2010.
- Alexander F, Pozharskii AS, Katritzky AR. Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry and Applications. 2nd ed John Wiley & Sons; Chichester, U.K.: 2011.
- Baumann M, Baxendale IR. Beilstein J. Org. Chem. 2013;9:2265. - PMC - PubMed
-
- Liu M, Bryant MS, Chen J, Lee S, Yaremko B, Li Z, Dell J, Lipari P, Malkowski M, Prioli N, Rossman RR, Korfmacher WA, Nomeir AA, Lin CC, Mallams AK, Doll RJ, Catino JJ, Girijavallabhan VM, Kirschmeier P, Bishop WR. Cancer Chemother. Pharmacol. 1998;43:50. - PubMed
-
- Chern J-H, Shia K-S, Hsu T-A, Tai C-L, Lee C-C, Lee Y-C, Chang C-S, Tseng S-N, Shih S-R. Bioorg. Med. Chem. Lett. 2004;14:2519. - PubMed
-
- Summa V, Petrocchi A, Matassa VG, Gardelli C, Muraglia E, Rowley M, Paz OG, Laufer R, Monteagudo E, Pace P. J. Med. Chem. 2006;49:6646. - PubMed
-
- Anagnostopulos H, Bartlett RR, Elben U, Stoll P. Eur. J. Med. Chem. 1989;24:227.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

