Lithium Carbonate in the Treatment of Graves' Disease with ATD-Induced Hepatic Injury or Leukopenia
- PMID: 26576153
- PMCID: PMC4630389
- DOI: 10.1155/2015/694023
Lithium Carbonate in the Treatment of Graves' Disease with ATD-Induced Hepatic Injury or Leukopenia
Abstract
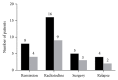
Objective. GD with ATD-induced hepatic injury or leukopenia occurs frequently in clinical practice. The purpose of the present study was to observe the clinical effect of lithium carbonate on hyperthyroidism in patients with GD with hepatic injury or leukopenia. Methods. Fifty-one patients with GD with hepatic injury or leukopenia participated in the study. All patients were treated with lithium carbonate, in addition to hepatoprotective drugs or drugs that increase white blood cell count. Thyroid function, liver function, and white blood cells were measured. Clinical outcomes were observed after a 1-year follow-up. Results. After treatment for 36 weeks, symptoms of hyperthyroidism and the level of thyroid hormones were improved and liver function, and white blood cells returned to a normal level. Twelve patients (23.5%) obtained clinical remission, 6 patients (11.8%) relapsed after withdrawal, 25 patients (49.0%) received radioiodine therapy, and 8 patients (15.7%) underwent surgical procedures after lithium carbonate treatment. Conclusion. Lithium carbonate has effects on the treatment of mild-to-moderate hyperthyroidism caused by GD, and it is particularly suitable for patients with ATD-induced hepatic injury or leukopenia.
Figures

References
-
- Williams K. V., Nayak S., Becker D., Reyes J., Burmeister L. A. Fifty years of experience with propylthiouracil-associated hepatotoxicity: what have we learned? The Journal of Clinical Endocrinology and Metabolism. 1997;82(6):1727–1733. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

