Activation of Two Sequential H-transfers in the Thymidylate Synthase Catalyzed Reaction
- PMID: 26576323
- PMCID: PMC4643671
- DOI: 10.1021/acscatal.5b01332
Activation of Two Sequential H-transfers in the Thymidylate Synthase Catalyzed Reaction
Abstract
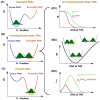
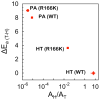
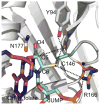
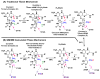
Thymidylate synthase (TSase) catalyzes the de novo biosynthesis of thymidylate, a precursor for DNA, and is thus an important target for chemotherapeutics and antibiotics. Two sequential C-H bond cleavages catalyzed by TSase are of particular interest: a reversible proton abstraction from the 2'-deoxy-uridylate substrate, followed by an irreversible hydride transfer forming the thymidylate product. QM/MM calculations of the former predicted a mechanism where the abstraction of the proton leads to formation of a novel nucleotide-folate intermediate that is not covalently bound to the enzyme (Wang, Z.; Ferrer, S.; Moliner, V.; Kohen, A. Biochemistry2013, 52, 2348-2358). Existence of such intermediate would hold promise as a target for a new class of drugs. Calculations of the subsequent hydride transfer predicted a concerted H-transfer and elimination of the enzymatic cysteine (Kanaan, N.; Ferrer, S.; Marti, S.; Garcia-Viloca, M.; Kohen, A.; Moliner, V. J. Am. Chem. Soc.2011, 133, 6692-6702). A key to both C-H activations is a highly conserved arginine (R166) that stabilizes the transition state of both H-transfers. Here we test these predictions by studying the R166 to lysine mutant of E. coli TSase (R166K) using intrinsic kinetic isotope effects (KIEs) and their temperature dependence to assess effects of the mutation on both chemical steps. The findings confirmed the predictions made by the QM/MM calculations, implicate R166 as an integral component of both reaction coordinates, and thus provide critical support to the nucleotide-folate intermediate as a new target for rational drug design.
Keywords: C-H bond activation; Donor and acceptor distances; Kinetic Isotope Effect; Phenomenological models; QM/MM calculations; Thymidylate Synthase; Tunneling ready state.
Figures



References
-
- Carosati E, Tochowicz A, Marverti G, Guaitoli G, Benedetti P, Ferrari S, Stroud RM, Finer-Moore J, Luciani R, Farina D, Cruciani G, Costi MP. J Med Chem. 2012;55:10272–10276. - PubMed
-
- Sergeeva OA, Khambatta HG, Cathers BE, Sergeeva MV. Biochem Biophys Res Commun. 2003;307:297–300. - PubMed
-
- Costi MP, Tondi D, Rinaldi M, Barlocco D, Pecorari P, Soragni F, Venturelli A, Stroud RM. Biochim Biophys Acta, Mol Basis Dis. 2002;1587:206–214. - PubMed
-
- Popat S, Matakidou A, Houlston RS. J Clin Oncol. 2004;22:529–536. - PubMed
-
- Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman L, Diasio RB, Danenberg PV. Clin Cancer Res. 2000;6:1322–1327. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
