Trends in clinical development timeframes for antiviral drugs launched in the UK, 1981-2014: a retrospective observational study
- PMID: 26576812
- PMCID: PMC4654359
- DOI: 10.1136/bmjopen-2015-009333
Trends in clinical development timeframes for antiviral drugs launched in the UK, 1981-2014: a retrospective observational study
Abstract
Objectives: Recent decades have witnessed the development of highly innovative new antiviral drug therapies. However, there are concerns that rising costs and lengthening development times could have implications for future patient access to innovative new drugs. We sought to establish whether the time taken for the clinical development of new antiviral drugs launched in the UK had increased since the 1980s.
Design and setting: Retrospective observational study of all new antiviral drugs licensed for use in the UK.
Primary and secondary outcome measures: Duration of clinical development (from initiation of studies in humans to receipt of Marketing Authorisation), subdivided into clinical trial and regulatory approval periods by the date of Marketing Authorisation Application.
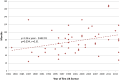
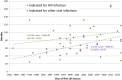
Results: 48 new antiviral drugs were licensed for use in the UK between 1981 and 2014 (inclusive), over half (54%) initially for HIV infection. The overall mean duration of clinical development was 77.2 months, of which 64.6 months was spent in clinical trials before regulatory submission. The total time in clinical development increased from 41.7 months for drugs licensed 1981-1992 to 91.7 months for drugs licensed 2004-2014. This increase was accounted for by an increase in the clinical trials period and not the regulatory approval period, for which there was no observable trend. Drugs initially licensed to treat hepatitis C had a longer duration of clinical development than those indicated for other viral infections. However, the, initially shorter clinical development durations of drugs indicated for HIV infection increased more rapidly across the study period than those indicated for other viral infections.
Conclusions: The time spent by antiviral drugs in clinical development has increased markedly in recent decades despite many initiatives to speed access to innovative new drugs. However, this represents only one part of the translational research pathway, and a complete picture of development timeframes is lacking.
Keywords: Drug development timeframes; Drug licensing; United Kingdom; Viral infection.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
Similar articles
-
Innovation and trends in the development and approval of antiviral medicines: 1987-2017 and beyond.Antiviral Res. 2018 Jul;155:76-88. doi: 10.1016/j.antiviral.2018.05.005. Epub 2018 May 15. Antiviral Res. 2018. PMID: 29758235 Free PMC article. Review.
-
Mismatched double-stranded RNA: polyI:polyC12U.Drugs R D. 2004;5(5):297-304. doi: 10.2165/00126839-200405050-00006. Drugs R D. 2004. PMID: 15357629 Free PMC article.
-
How innovative are new drugs launched in the UK? A retrospective study of new drugs listed in the British National Formulary (BNF) 2001-2012.BMJ Open. 2014 Oct 24;4(10):e006235. doi: 10.1136/bmjopen-2014-006235. BMJ Open. 2014. PMID: 25344485 Free PMC article.
-
Trends in the development and approval of monoclonal antibodies for viral infections.BioDrugs. 2007;21(1):1-7. doi: 10.2165/00063030-200721010-00001. BioDrugs. 2007. PMID: 17263584 Free PMC article.
-
Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs.Int J Biol Macromol. 2021 Mar 1;172:524-541. doi: 10.1016/j.ijbiomac.2021.01.076. Epub 2021 Jan 14. Int J Biol Macromol. 2021. PMID: 33454328 Free PMC article. Review.
Cited by
-
Development of a rapid image-based high-content imaging screening assay to evaluate therapeutic antibodies against the monkeypox virus.Antiviral Res. 2023 Feb;210:105513. doi: 10.1016/j.antiviral.2022.105513. Epub 2022 Dec 30. Antiviral Res. 2023. PMID: 36592670 Free PMC article.
-
A review on immunoglobulin Y (IgY) conjugated with metal nanoparticles and biomedical uses.Bioprocess Biosyst Eng. 2023 Nov;46(11):1533-1538. doi: 10.1007/s00449-023-02909-x. Epub 2023 Jul 26. Bioprocess Biosyst Eng. 2023. PMID: 37493807 Review.
-
Immunoglobulin Y for Potential Diagnostic and Therapeutic Applications in Infectious Diseases.Front Immunol. 2021 Jun 9;12:696003. doi: 10.3389/fimmu.2021.696003. eCollection 2021. Front Immunol. 2021. PMID: 34177963 Free PMC article. Review.
-
Identification of broad anti-coronavirus chemical agents for repurposing against SARS-CoV-2 and variants of concern.Curr Res Virol Sci. 2022;3:100019. doi: 10.1016/j.crviro.2022.100019. Epub 2022 Jan 15. Curr Res Virol Sci. 2022. PMID: 35072124 Free PMC article.
-
SARS-CoV-2-specific immunoglobulin Y antibodies are protective in infected mice.PLoS Pathog. 2022 Sep 19;18(9):e1010782. doi: 10.1371/journal.ppat.1010782. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36121829 Free PMC article.
References
-
- House of Commons Select Committee on Health. The influence of the pharmaceutical industry. London: House of Commons, 2005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical