Differential immunogenicity between HAdV-5 and chimpanzee adenovirus vector ChAdOx1 is independent of fiber and penton RGD loop sequences in mice
- PMID: 26576856
- PMCID: PMC4649739
- DOI: 10.1038/srep16756
Differential immunogenicity between HAdV-5 and chimpanzee adenovirus vector ChAdOx1 is independent of fiber and penton RGD loop sequences in mice
Abstract
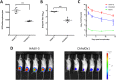
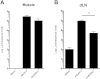
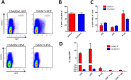
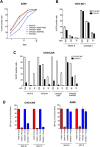
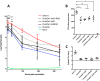
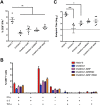
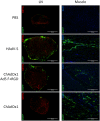
Replication defective adenoviruses are promising vectors for the delivery of vaccine antigens. However, the potential of a vector to elicit transgene-specific adaptive immune responses is largely dependent on the viral serotype used. HAdV-5 (Human adenovirus C) vectors are more immunogenic than chimpanzee adenovirus vectors from species Human adenovirus E (ChAdOx1 and AdC68) in mice, though the mechanisms responsible for these differences in immunogenicity remain poorly understood. In this study, superior immunogenicity was associated with markedly higher levels of transgene expression in vivo, particularly within draining lymph nodes. To investigate the viral factors contributing to these phenotypes, we generated recombinant ChAdOx1 vectors by exchanging components of the viral capsid reported to be principally involved in cell entry with the corresponding sequences from HAdV-5. Remarkably, pseudotyping with the HAdV-5 fiber and/or penton RGD loop had little to no effect on in vivo transgene expression or transgene-specific adaptive immune responses despite considerable species-specific sequence heterogeneity in these components. Our results suggest that mechanisms governing vector transduction after intramuscular administration in mice may be different from those described in vitro.
Conflict of interest statement
MDJD, SCG, AVSH, and MGC are named inventors on a patent application describing the ChAdOx1 vector (US2015044766).
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

