Hydroxyproline O-arabinosyltransferase mutants oppositely alter tip growth in Arabidopsis thaliana and Physcomitrella patens
- PMID: 26577059
- PMCID: PMC4738400
- DOI: 10.1111/tpj.13079
Hydroxyproline O-arabinosyltransferase mutants oppositely alter tip growth in Arabidopsis thaliana and Physcomitrella patens
Abstract
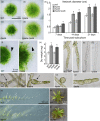
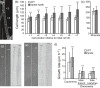
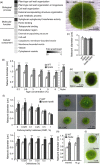
Hydroxyproline O-arabinosyltransferases (HPATs) are members of a small, deeply conserved family of plant-specific glycosyltransferases that add arabinose sugars to diverse proteins including cell wall-associated extensins and small signaling peptides. Recent genetic studies in flowering plants suggest that different HPAT homologs have been co-opted to function in diverse species-specific developmental contexts. However, nothing is known about the roles of HPATs in basal plants. We show that complete loss of HPAT function in Arabidopsis thaliana and the moss Physcomitrella patens results in a shared defect in gametophytic tip cell growth. Arabidopsis hpat1/2/3 triple knockout mutants suffer from a strong male sterility defect as a consequence of pollen tubes that fail to fully elongate following pollination. Knocking out the two HPAT genes of Physcomitrella results in larger multicellular filamentous networks due to increased elongation of protonemal tip cells. Physcomitrella hpat mutants lack cell-wall associated hydroxyproline arabinosides and can be rescued with exogenous cellulose, while global expression profiling shows that cell wall-associated genes are severely misexpressed, implicating a defect in cell wall formation during tip growth. Our findings point to a major role for HPATs in influencing cell elongation during tip growth in plants.
Keywords: Arabidopsis thaliana; Physcomitrella patens; arabinosylation; cell wall; development; extensins; glycosylation; pollination; protonema; tip growth.
© 2015 The Authors The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Figures


Similar articles
-
Exocyst mutants suppress pollen tube growth and cell wall structural defects of hydroxyproline O-arabinosyltransferase mutants.Plant J. 2020 Aug;103(4):1399-1419. doi: 10.1111/tpj.14808. Epub 2020 Jun 12. Plant J. 2020. PMID: 32391581 Free PMC article.
-
Identification of three hydroxyproline O-arabinosyltransferases in Arabidopsis thaliana.Nat Chem Biol. 2013 Nov;9(11):726-30. doi: 10.1038/nchembio.1351. Epub 2013 Sep 15. Nat Chem Biol. 2013. PMID: 24036508
-
Partial functional conservation of IRX10 homologs in physcomitrella patens and Arabidopsis thaliana indicates an evolutionary step contributing to vascular formation in land plants.BMC Plant Biol. 2013 Jan 3;13:3. doi: 10.1186/1471-2229-13-3. BMC Plant Biol. 2013. PMID: 23286876 Free PMC article.
-
Structure and functional analysis of the 26S proteasome subunits from plants.Mol Biol Rep. 1999 Apr;26(1-2):137-46. doi: 10.1023/a:1006926322501. Mol Biol Rep. 1999. PMID: 10363660 Review.
-
Understanding myosin functions in plants: are we there yet?Curr Opin Plant Biol. 2013 Dec;16(6):710-7. doi: 10.1016/j.pbi.2013.10.004. Curr Opin Plant Biol. 2013. PMID: 24446546 Review.
Cited by
-
The hydroxyproline O-arabinosyltransferase FIN4 is required for tomato pollen intine development.Plant Reprod. 2023 Jun;36(2):173-191. doi: 10.1007/s00497-023-00459-6. Epub 2023 Feb 7. Plant Reprod. 2023. PMID: 36749417
-
Plant Protein O-Arabinosylation.Front Plant Sci. 2021 Mar 18;12:645219. doi: 10.3389/fpls.2021.645219. eCollection 2021. Front Plant Sci. 2021. PMID: 33815452 Free PMC article. Review.
-
SEC1A is a major Arabidopsis Sec1/Munc18 gene in vesicle trafficking during pollen tube tip growth.Plant J. 2022 Jun;110(5):1353-1369. doi: 10.1111/tpj.15742. Epub 2022 Apr 6. Plant J. 2022. PMID: 35306707 Free PMC article.
-
Dual CLAVATA3 Peptides in Arabidopsis Shoot Stem Cell Signaling.J Plant Biol. 2017 Oct;60(5):506-512. doi: 10.1007/s12374-017-0083-2. Epub 2017 Oct 6. J Plant Biol. 2017. PMID: 30310351 Free PMC article.
-
O-glycosylation of the extracellular domain of pollen class I formins modulates their plasma membrane mobility.J Exp Bot. 2022 Jun 24;73(12):3929-3945. doi: 10.1093/jxb/erac131. J Exp Bot. 2022. PMID: 35383367 Free PMC article.
References
-
- Al‐Shahrour, F. , Díaz‐Uriarte, R. and Dopazo, J. (2004) FatiGO: a web tool for finding significant associations of Gene Ontology terms with groups of genes. Bioinformatics, 20, 578–580. - PubMed
-
- Aoyama, T. , Hiwatashi, Y. , Shigyo, M. , Kofuji, R. , Kubo, M. , Ito, M. and Hasebe, M. (2012) AP2‐type transcription factors determine stem cell identity in the moss Physcomitrella patens . Development, 139, 3120–3129. - PubMed
-
- Baumberger, N. , Steiner, M. , Ryser, U. , Keller, B. and Ringli, C. (2003a) Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J. 35, 71–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

