Bony labyrinth shape variation in extant Carnivora: a case study of Musteloidea
- PMID: 26577069
- PMCID: PMC5341543
- DOI: 10.1111/joa.12421
Bony labyrinth shape variation in extant Carnivora: a case study of Musteloidea
Abstract
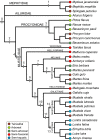
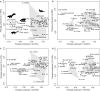
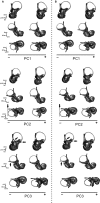
The bony labyrinth provides a proxy for the morphology of the inner ear, a primary cognitive organ involved in hearing, body perception in space, and balance in vertebrates. Bony labyrinth shape variations often are attributed to phylogenetic and ecological factors. Here we use three-dimensional (3D) geometric morphometrics to examine the phylogenetic and ecological patterns of variation in the bony labyrinth morphology of the most species-rich and ecologically diversified traditionally recognized superfamily of Carnivora, the Musteloidea (e.g. weasels, otters, badgers, red panda, skunks, raccoons, coatis). We scanned the basicrania of specimens belonging to 31 species using high-resolution X-ray computed micro-tomography (μCT) to virtually reconstruct 3D models of the bony labyrinths. Labyrinth morphology is captured by a set of six fixed landmarks on the vestibular and cochlear systems, and 120 sliding semilandmarks, slid at the center of the semicircular canals and the cochlea. We found that the morphology of this sensory structure is not significantly influenced by bony labyrinth size, in comparisons across all musteloids or in any of the individual traditionally recognized families (Mephitidae, Procyonidae, Mustelidae). PCA (principal components analysis) of shape data revealed that bony labyrinth morphology is clearly distinguishable between musteloid families, and permutation tests of the Kmult statistic confirmed that the bony labyrinth shows a phylogenetic signal in musteloids and in most mustelids. Both the vestibular and cochlear regions display morphological differences among the musteloids sampled, associated with the size and curvature of the semicircular canals, angles between canals, presence or absence of a secondary common crus, degree of lateral compression of the vestibule, orientation of the cochlea relative to the semicircular canals, proportions of the cochlea, and degree of curvature of its turns. We detected a significant ecological signal in the bony labyrinth shape of musteloids, differentiating semi-aquatic taxa from non-aquatic ones (the taxa assigned to terrestrial, arboreal, semi-arboreal, and semi-fossorial categories), and a significant signal for mustelids, differentiating the bony labyrinths of terrestrial, semi-arboreal, arboreal, semi-fossorial and semi-aquatic species from each other. Otters and minks are distinguished from non-aquatic musteloids by an oval rather than circular anterior canal, sinuous rather than straight lateral canal, and acute rather than straight angle between the posterior and lateral semicircular canals - each of these morphological characters has been related previously to animal sensitivity for detecting head motion in space.
Keywords: Musteloidea; allometry; inner ear; locomotion; morphology; phylogeny; semilandmark sliding; three-dimensional geometric morphometrics.
© 2015 Anatomical Society.
Figures




Similar articles
-
Deciphering and dating the red panda's ancestry and early adaptive radiation of Musteloidea.Mol Phylogenet Evol. 2009 Dec;53(3):907-22. doi: 10.1016/j.ympev.2009.08.019. Epub 2009 Aug 21. Mol Phylogenet Evol. 2009. PMID: 19699810
-
Shape variation and ontogeny of the ruminant bony labyrinth, an example in Tragulidae.J Anat. 2016 Sep;229(3):422-35. doi: 10.1111/joa.12487. Epub 2016 Jun 1. J Anat. 2016. PMID: 27245372 Free PMC article.
-
Comparative analysis of vestibular ecomorphology in birds.J Anat. 2017 Dec;231(6):990-1018. doi: 10.1111/joa.12726. J Anat. 2017. PMID: 29156494 Free PMC article.
-
Comparative review of the human bony labyrinth.Am J Phys Anthropol. 1998;Suppl 27:211-51. doi: 10.1002/(sici)1096-8644(1998)107:27+<211::aid-ajpa8>3.3.co;2-m. Am J Phys Anthropol. 1998. PMID: 9881527 Review.
-
Form and function of the mammalian inner ear.J Anat. 2016 Feb;228(2):324-37. doi: 10.1111/joa.12308. Epub 2015 Apr 25. J Anat. 2016. PMID: 25911945 Free PMC article. Review.
Cited by
-
First Attempt to Infer Sound Hearing and Its Paleoenvironmental Implications in the Extinct Insular Canid Cynotherium sardous Studiati, 1857 (Sardinia, Italy).Animals (Basel). 2022 Mar 25;12(7):833. doi: 10.3390/ani12070833. Animals (Basel). 2022. PMID: 35405823 Free PMC article.
-
Emergent network properties link phenotypic modules to ecomorphological divergence in carnivoran mammals.iScience. 2025 Jan 16;28(2):111828. doi: 10.1016/j.isci.2025.111828. eCollection 2025 Feb 21. iScience. 2025. PMID: 39967867 Free PMC article.
-
Evolutionary Specializations in the Venous Anatomy of the Two-Toed Sloth (Choloepus didactylus): Insights from CT-scan 3D Reconstructions.Animals (Basel). 2024 Jun 12;14(12):1768. doi: 10.3390/ani14121768. Animals (Basel). 2024. PMID: 38929387 Free PMC article.
-
Evolution of arboreality and fossoriality in squirrels and aplodontid rodents: Insights from the semicircular canals of fossil rodents.J Anat. 2021 Jan;238(1):96-112. doi: 10.1111/joa.13296. Epub 2020 Aug 19. J Anat. 2021. PMID: 32812227 Free PMC article.
-
Locomotor behavior and hearing sensitivity in an early lagomorph reconstructed from the bony labyrinth.Ecol Evol. 2023 Mar 18;13(3):e9890. doi: 10.1002/ece3.9890. eCollection 2023 Mar. Ecol Evol. 2023. PMID: 36942029 Free PMC article.
References
-
- Adams DC (2014) A generalized K statistic for estimating phylogenetic signal from shape and other high‐dimensional multivariate data. Syst Biol 65, 685–697. - PubMed
-
- Adams DC, Otarola‐Castillo E (2013) geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol 4, 393–399.
-
- Adams DC, Collyer ML, Sherratt E (2015) geomorph: software for geometric morphometric analyses. R package version 2.1.4. http://cran.r-project.org/web/packages/geomorph/index.html.
-
- Alloing‐Seguier L, Sanchez‐Villagra MR, Lee MSY, et al. (2013) The bony labyrinth in diprotodontian marsupial mammals: diversity in extant and extinct forms and relationships with size and phylogeny. J Mamm Evol 20, 191–198.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

