Engineering Corynebacterium glutamicum to produce 5-aminolevulinic acid from glucose
- PMID: 26577071
- PMCID: PMC4650169
- DOI: 10.1186/s12934-015-0364-8
Engineering Corynebacterium glutamicum to produce 5-aminolevulinic acid from glucose
Abstract
Background: Corynebacterium glutamicum is generally regarded as a safe microorganism and is used to produce many biochemicals, including L-glutamate. 5-Aminolevulinic acid (ALA) is an L-glutamate derived non-protein amino acid, and is widely applied in fields such as medicine and agriculture.
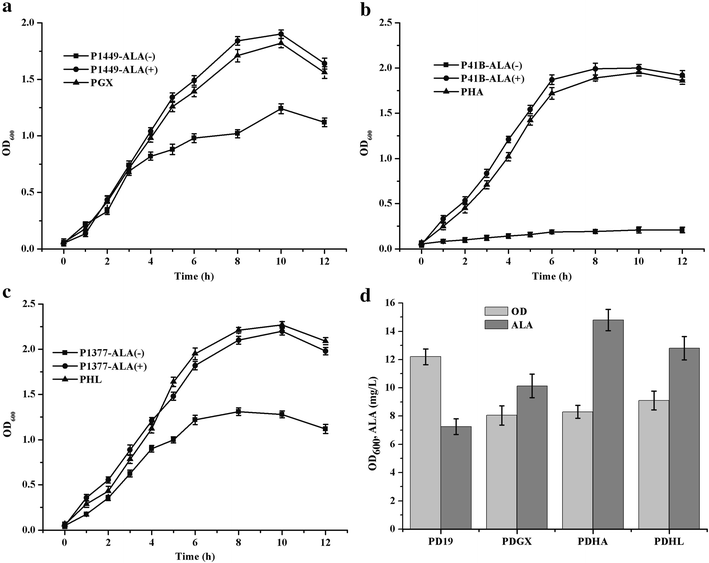
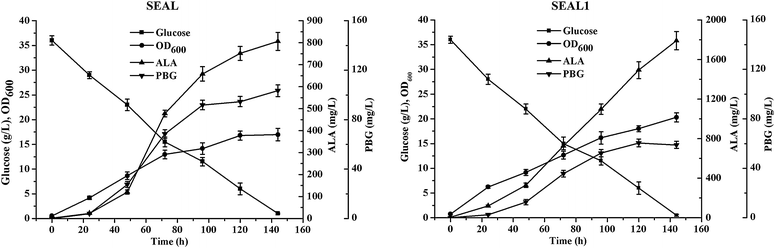
Results: The products of the gltX, hemA, and hemL genes participate in the synthesis of ALA from L-glutamate. Their annotated C. glutamicum homologs were shown to be functional using heterologous complementation and overexpression techniques. Coexpression of hemA and hemL in native host led to the accumulation of ALA, suggesting the potential of C. glutamicum to produce ALA for research and commercial purposes. To improve ALA production, we constructed recombinant C. glutamicum strains expressing hemA and hemL derived from different organisms. Transcriptome analysis indicated that the dissolved oxygen level and Fe(2+) concentration had major effects on ALA synthesis. The downstream pathway of heme biosynthesis was inhibited using small molecules or introducing genetic modifications. Small-scale flask cultures of engineered C. glutamicum produced 1.79 g/L of ALA.
Conclusion: Functional characterization of the key enzymes indicated complex regulation of the heme biosynthetic pathway in C. glutamicum. Systematic analysis and molecular genetic engineering of C. glutamicum may facilitate its development as a system for large-scale synthesis of ALA.
Figures


References
-
- Kinoshita S, Udaka S, Shimono M. Studies on the amino acid fermentation. Part 1. Production of l-glutamic acid by various microorganisms. J Gen Appl Microbiol. 2004;50:331–343. - PubMed
-
- Mike LA, Dutter BF, Stauff DL, Moore JL, Vitkoe NP, Aranmolate O, Kehl-Fie TE, Sullivan S, Reid PR, DuBois JL, Richardson AR, Caprioli RM, Sulikowski GA, Skaar EP. Activation of heme biosynthesis by a small molecule that is toxic to fermenting Staphylococcus aureus. Proc Natl Acad Sci. 2013;110:8206–8211. doi: 10.1073/pnas.1303674110. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

