Innate immunity against molecular mimicry: Examining galectin-mediated antimicrobial activity
- PMID: 26577077
- PMCID: PMC7428859
- DOI: 10.1002/bies.201500055
Innate immunity against molecular mimicry: Examining galectin-mediated antimicrobial activity
Abstract
Adaptive immunity provides the unique ability to respond to a nearly infinite range of antigenic determinants. Given the inherent plasticity of the adaptive immune system, a series of tolerance mechanisms exist to reduce reactivity toward self. While this reduces the probability of autoimmunity, it also creates an important gap in adaptive immunity: the ability to recognize microbes that look like self. As a variety of microbes decorate themselves in self-like carbohydrate antigens and tolerance reduces the ability of adaptive immunity to react with self-like structures, protection against molecular mimicry likely resides within the innate arm of immunity. In this review, we will explore the potential consequences of microbial molecular mimicry, including factors within innate immunity that appear to specifically target microbes expressing self-like antigens, and therefore provide protection against molecular mimicry.
Keywords: adaptive immunity; carbohydrates; galectins; innate immunity; lectins; microbes.
© 2015 WILEY Periodicals, Inc.
Figures
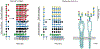
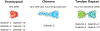
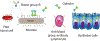
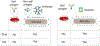

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

