Strategy and rationale for urine collection protocols employed in the NEPTUNE study
- PMID: 26577187
- PMCID: PMC4650313
- DOI: 10.1186/s12882-015-0185-3
Strategy and rationale for urine collection protocols employed in the NEPTUNE study
Abstract
Background: Glomerular diseases are potentially fatal, requiring aggressive interventions and close monitoring. Urine is a readily-accessible body fluid enriched in molecular signatures from the kidney and therefore particularly suited for routine clinical analysis as well as development of non-invasive biomarkers for glomerular diseases.
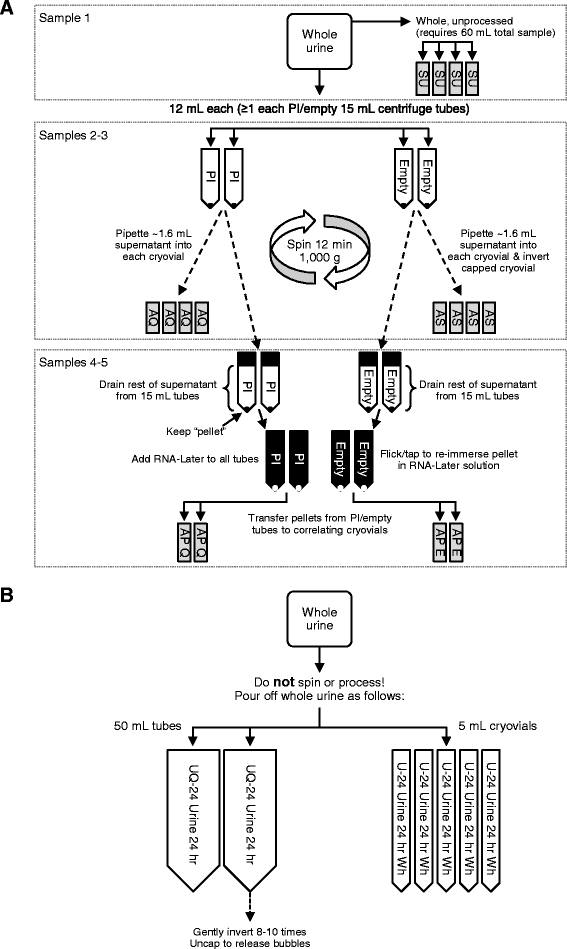
Methods: The Nephrotic Syndrome Study Network (NEPTUNE; ClinicalTrials.gov Identifier NCT01209000) is a North American multicenter collaborative consortium established to develop a translational research infrastructure for nephrotic syndrome. This includes standardized urine collections across all participating centers for the purpose of discovering non-invasive biomarkers for patients with nephrotic syndrome due to minimal change disease, focal segmental glomerulosclerosis, and membranous nephropathy. Here we describe the organization and methods of urine procurement and banking procedures in NEPTUNE.
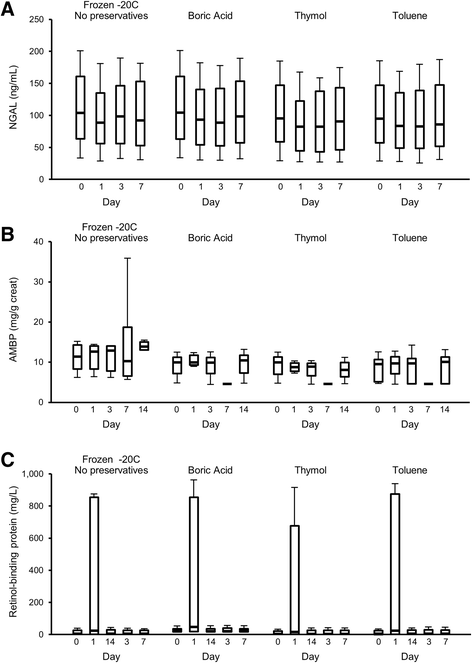
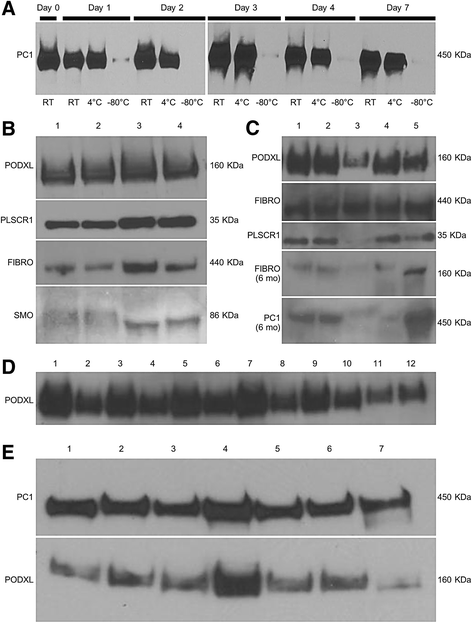
Results: We discuss the rationale for urine collection and storage conditions, and demonstrate the performance of three experimental analytes (neutrophil gelatinase-associated lipocalin [NGAL], retinol binding globulin, and alpha-1 microglobulin) under these conditions with and without urine preservatives (thymol, toluene, and boric acid). We also demonstrate the quality of RNA and protein collected from the urine cellular pellet and exosomes.
Conclusions: The urine collection protocol in NEPTUNE allows robust detection of a wide range of proteins and RNAs from urine supernatant and pellets collected longitudinally from each patient over 5 years. Combined with the detailed clinical and histopathologic data, this provides a unique resource for exploration and validation of new or accepted markers of glomerular diseases.
Trial registration: ClinicalTrials.gov Identifier NCT01209000.
Figures
References
-
- Standard Protocol for Urine Collection and Storage [http://www.hkupp.org/Urine%20collectiion%20Documents.htm]. Access date 11/15/2015.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

