Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise
- PMID: 26577795
- PMCID: PMC4705137
- DOI: 10.1007/s00125-015-3795-1
Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise
Abstract
Aims/hypothesis: Additional safe and effective therapies for type 2 diabetes are needed, especially ones that do not cause weight gain and have a low risk of hypoglycaemia. The present study evaluated albiglutide as monotherapy.
Methods: In this placebo-controlled study, 309 patients (aged ≥ 18 years) with type 2 diabetes inadequately controlled by diet and exercise and who were not using a glucose-lowering agent (HbA1c 7.0-10.0% [53.00-85.79 mmol/mol], body mass index 20-45 kg/m(2), and fasting C-peptide ≥ 0.26 nmol/l) were randomised (1:1:1 on a fixed randomisation schedule using an interactive voice response system) to receive once-weekly albiglutide 30 mg (n = 102) or 50 mg (n = 102) or matching placebo (n = 105). The study treatments were blinded to both patients and study personnel. All study data were collected at individual patient clinic visits. The primary efficacy endpoint was change in HbA1c from baseline to week 52. The primary analysis was applied to the intent-to-treat population. Additional efficacy and safety endpoints were assessed.
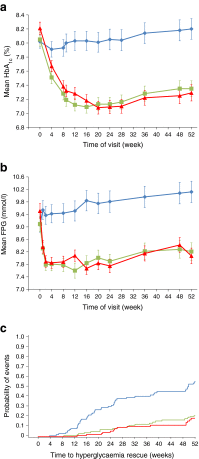
Results: At week 52, both albiglutide 30 mg and 50 mg were superior to placebo in reducing HbA1c. The least-squares means treatment difference from placebo was -0.84% (95% CI -1.11%, -0.58%; p < 0.0001) with albiglutide 30 mg and -1.04% (-1.31%, -0.77%; p < 0.0001) with albiglutide 50 mg. Injection-site reactions were reported more frequently with albiglutide (30 mg: 17.8%; 50 mg: 22.2%) than with placebo (9.9%). Other commonly reported adverse events included nausea, diarrhoea, vomiting and hypoglycaemia; the incidences of these were generally similar across treatment groups.
Conclusions/interpretation: Albiglutide is safe and effective as monotherapy and significantly lowered HbA1c levels over 52 weeks, did not cause weight gain, and had good gastrointestinal tolerability and a low rate of hypoglycaemia compared with placebo. Trial registration ClinicalTrials.gov NCT00849017 Funding This study was sponsored by GlaxoSmithKline.
Keywords: Albiglutide; GLP-1 agonist; Randomised controlled trial; Type 2 diabetes.
Figures
References
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. - DOI - PubMed
-
- Esposito K, Chiodini P, Bellastella G, Maiorino MI, Giugliano D. Proportion of patients at HbA1c target <7% with eight classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78 945 patients. Diabetes Obes Metab. 2012;14:228–233. doi: 10.1111/j.1463-1326.2011.01512.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

