The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer--a systematic review
- PMID: 26578067
- PMCID: PMC4650834
- DOI: 10.1186/s13058-015-0648-2
The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer--a systematic review
Abstract
Introduction: This study aimed at evaluating the overall survival (OS) gain associated with human epidermal growth factor receptor 2 (HER2)-directed therapies in patients with metastatic breast cancer (mBC).
Methods: A bibliographic search was conducted in PubMed and Cochrane databases. Only phase III randomized controlled trials (RCTs) including HER2-positive (HER2+) mBC patients were included in this review. OS was defined as time from randomization until the occurrence of death from any cause. Studies have been grouped according to the line of treatment, i.e., first-line or second-line or beyond.
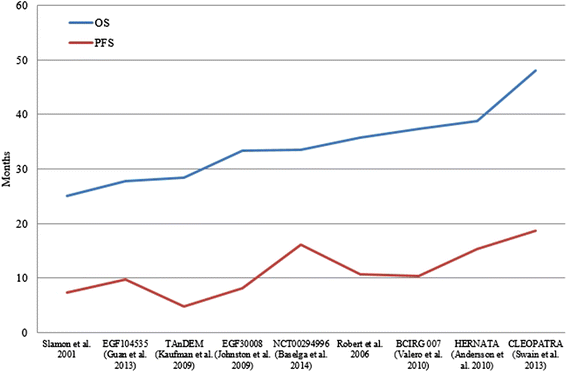
Results: Nineteen RCTs were eligible for inclusion, of which 12 assessed therapies targeting HER2+ mBC in the first-line setting. OS improved from 20.3 months in the first RCT (standard chemotherapy; Slamon et al. (N Engl J Med 344:783-92, 2001)) evaluating HER2-targeting therapies to 48 months in the study of Swain et al. (Lancet Oncol 14:461-71, 2013), with triple combination of pertuzumab, trastuzumab and docetaxel. Seven RCTs evaluated the OS of HER2-targeting therapies in the second-line setting and beyond. The OS in second-line setting improved from 15.3 months (capecitabine; Cameron et al. (Breast Cancer Res Treat 112:533-43, 2008)) to 30.7 months (trastuzumab emtansine; Verma et al. (N Engl J Med 367:1783-91, 2012)). In the third-line setting, the association of lapatinib and trastuzumab has demonstrated to improve OS to 4.5 months compared with lapatinib alone (14 months vs. 9.5 months; Blackwell et al. (J Clin Oncol 30:2585-92, 2012)).
Conclusions: HER2-directed therapies had an undeniable beneficial impact on the OS of patients with HER2+ mBC. The triple combination of docetaxel, pertuzumab and trastuzumab is associated with a survival extent of more than 4.5 years, compared with a life expectancy of 1.5 years achieved 14 years ago.
Figures

References
-
- Swain SM, Kim SB, Cortés J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–71. doi: 10.1016/S1470-2045(13)70130-X. - DOI - PMC - PubMed
-
- Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–43. doi: 10.1007/s10549-007-9885-0. - DOI - PubMed
-
- Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol. 2012;30:2585–92. doi: 10.1200/JCO.2011.35.6725. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

