Antibody Response to Serpin B13 Induces Adaptive Changes in Mouse Pancreatic Islets and Slows Down the Decline in the Residual Beta Cell Function in Children with Recent Onset of Type 1 Diabetes Mellitus
- PMID: 26578518
- PMCID: PMC4697161
- DOI: 10.1074/jbc.M115.687848
Antibody Response to Serpin B13 Induces Adaptive Changes in Mouse Pancreatic Islets and Slows Down the Decline in the Residual Beta Cell Function in Children with Recent Onset of Type 1 Diabetes Mellitus
Abstract
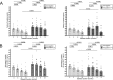
Type 1 diabetes mellitus (T1D) is characterized by a heightened antibody (Ab) response to pancreatic islet self-antigens, which is a biomarker of progressive islet pathology. We recently identified a novel antibody to clade B serpin that reduces islet-associated T cell accumulation and is linked to the delayed onset of T1D. As natural immunity to clade B arises early in life, we hypothesized that it may influence islet development during that time. To test this possibility healthy young Balb/c male mice were injected with serpin B13 mAb or IgG control and examined for the number and cellularity of pancreatic islets by immunofluorescence and FACS. Beta cell proliferation was assessed by measuring nucleotide analog 5-ethynyl-2'-deoxyuridine (5-EdU) incorporation into the DNA and islet Reg gene expression was measured by real time PCR. Human studies involved measuring anti-serpin B13 autoantibodies by Luminex. We found that injecting anti-serpin B13 monoclonal Ab enhanced beta cell proliferation and Reg gene expression, induced the generation of ∼80 pancreatic islets per animal, and ultimately led to increase in the beta cell mass. These findings are relevant to human T1D because our analysis of subjects just diagnosed with T1D revealed an association between baseline anti-serpin activity and slower residual beta cell function decline in the first year after the onset of diabetes. Our findings reveal a new role for the anti-serpin immunological response in promoting adaptive changes in the endocrine pancreas and suggests that enhancement of this response could potentially help impede the progression of T1D in humans.
Keywords: Type 1 diabetes; antibody; beta cell (B-cell); pancreatic islet; serpin.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures








Similar articles
-
Cellular proliferation in mouse and human pancreatic islets is regulated by serpin B13 inhibition and downstream targeting of E-cadherin by cathepsin L.Diabetologia. 2019 May;62(5):822-834. doi: 10.1007/s00125-019-4834-0. Epub 2019 Mar 1. Diabetologia. 2019. PMID: 30824970
-
Anti-serpin antibody-mediated regulation of proteases in autoimmune diabetes.J Biol Chem. 2013 Jan 18;288(3):1612-9. doi: 10.1074/jbc.M112.409664. Epub 2012 Nov 29. J Biol Chem. 2013. PMID: 23195956 Free PMC article.
-
Islet remodeling in female mice with spontaneous autoimmune and streptozotocin-induced diabetes.PLoS One. 2014 Aug 7;9(8):e102843. doi: 10.1371/journal.pone.0102843. eCollection 2014. PLoS One. 2014. PMID: 25101835 Free PMC article.
-
Immunomodulation and regeneration of islet Beta cells by cytokines in autoimmune type 1 diabetes.J Interferon Cytokine Res. 2011 Oct;31(10):711-9. doi: 10.1089/jir.2011.0025. Epub 2011 Aug 18. J Interferon Cytokine Res. 2011. PMID: 21851268 Review.
-
Type I diabetes mellitus: a predictable autoimmune disease with interindividual variation in the rate of beta cell destruction.Clin Immunol Immunopathol. 1989 Jan;50(1 Pt 2):S85-95. doi: 10.1016/0090-1229(89)90115-3. Clin Immunol Immunopathol. 1989. PMID: 2642771 Review.
Cited by
-
Exocrine-Endocrine Crosstalk: The Influence of Pancreatic Cellular Communications on Organ Growth, Function and Disease.Front Endocrinol (Lausanne). 2022 Jun 13;13:904004. doi: 10.3389/fendo.2022.904004. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35769082 Free PMC article. Review.
-
The Role of Proteases and Serpin Protease Inhibitors in β-Cell Biology and Diabetes.Biomolecules. 2022 Jan 2;12(1):67. doi: 10.3390/biom12010067. Biomolecules. 2022. PMID: 35053215 Free PMC article. Review.
-
Exocrine pancreas proteases regulate β-cell proliferation in zebrafish ciliopathy models and in murine systems.Biol Open. 2021 Jun 15;10(6):bio046839. doi: 10.1242/bio.046839. Epub 2021 Jun 14. Biol Open. 2021. PMID: 34125181 Free PMC article.
-
Cellular proliferation in mouse and human pancreatic islets is regulated by serpin B13 inhibition and downstream targeting of E-cadherin by cathepsin L.Diabetologia. 2019 May;62(5):822-834. doi: 10.1007/s00125-019-4834-0. Epub 2019 Mar 1. Diabetologia. 2019. PMID: 30824970
-
Competing endogenous RNA network analysis explores the key lncRNAs, miRNAs, and mRNAs in type 1 diabetes.BMC Med Genomics. 2021 Feb 1;14(1):35. doi: 10.1186/s12920-021-00877-3. BMC Med Genomics. 2021. PMID: 33526014 Free PMC article.
References
-
- Karjalainen J., Salmela P., Ilonen J., Surcel H. M., and Knip M. (1989) Comparison of childhood and adult type I diabetes mellitus. N. Engl. J. Med. 320, 881–886 - PubMed
-
- Abts H. F., Welss T., Mirmohammadsadegh A., Köhrer K., Michel G., and Ruzicka T. (1999) Cloning and characterization of hurpin (protease inhibitor 13): a new skin-specific, UV- repressible serine proteinase inhibitor of the ovalbumin serpin family. J. Mol. Biol. 293, 29–39 - PubMed
-
- Welss T., Sun J., Irving J. A., Blum R., Smith A. I., Whisstock J. C., Pike R. N., von Mikecz A., and Ruzicka T., Bird P. I., and Abts H. F. (2003) Hurpin is a selective inhibitor of lysosomal cathepsin L and protects keratinocytes from ultraviolet induced apoptosis. Biochemistry 42, 7381–7389 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

