Binding dynamics of a monomeric SSB protein to DNA: a single-molecule multi-process approach
- PMID: 26578575
- PMCID: PMC4678828
- DOI: 10.1093/nar/gkv1225
Binding dynamics of a monomeric SSB protein to DNA: a single-molecule multi-process approach
Abstract
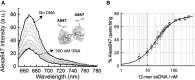
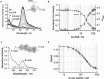
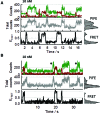
Single-stranded DNA binding proteins (SSBs) are ubiquitous across all organisms and are characterized by the presence of an OB (oligonucleotide/oligosaccharide/oligopeptide) binding motif to recognize single-stranded DNA (ssDNA). Despite their critical role in genome maintenance, our knowledge about SSB function is limited to proteins containing multiple OB-domains and little is known about single OB-folds interacting with ssDNA. Sulfolobus solfataricus SSB (SsoSSB) contains a single OB-fold and being the simplest representative of the SSB-family may serve as a model to understand fundamental aspects of SSB:DNA interactions. Here, we introduce a novel approach based on the competition between Förster resonance energy transfer (FRET), protein-induced fluorescence enhancement (PIFE) and quenching to dissect SsoSSB binding dynamics at single-monomer resolution. We demonstrate that SsoSSB follows a monomer-by-monomer binding mechanism that involves a positive-cooperativity component between adjacent monomers. We found that SsoSSB dynamic behaviour is closer to that of Replication Protein A than to Escherichia coli SSB; a feature that might be inherited from the structural analogies of their DNA-binding domains. We hypothesize that SsoSSB has developed a balance between high-density binding and a highly dynamic interaction with ssDNA to ensure efficient protection of the genome but still allow access to ssDNA during vital cellular processes.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
The structural basis of DNA binding by the single-stranded DNA-binding protein from Sulfolobus solfataricus.Biochem J. 2015 Jan 15;465(2):337-46. doi: 10.1042/BJ20141140. Biochem J. 2015. PMID: 25367669
-
Backbone and side-chain ¹H, ¹³C and ¹⁵N resonance assignments of the OB domain of the single stranded DNA binding protein from Sulfolobus solfataricus and chemical shift mapping of the DNA-binding interface.Biomol NMR Assign. 2014 Oct;8(2):243-6. doi: 10.1007/s12104-013-9492-4. Epub 2013 Jun 8. Biomol NMR Assign. 2014. PMID: 23749431
-
The single-stranded DNA binding protein of Sulfolobus solfataricus acts in the presynaptic step of homologous recombination.J Mol Biol. 2010 Mar 19;397(1):31-45. doi: 10.1016/j.jmb.2010.01.004. Epub 2010 Jan 18. J Mol Biol. 2010. PMID: 20080104
-
The mechanism of action of the SSB interactome reveals it is the first OB-fold family of genome guardians in prokaryotes.Protein Sci. 2021 Sep;30(9):1757-1775. doi: 10.1002/pro.4140. Epub 2021 Jun 14. Protein Sci. 2021. PMID: 34089559 Free PMC article. Review.
-
Multiple human single-stranded DNA binding proteins function in genome maintenance: structural, biochemical and functional analysis.Crit Rev Biochem Mol Biol. 2009 Jun;44(2-3):98-116. doi: 10.1080/10409230902849180. Crit Rev Biochem Mol Biol. 2009. PMID: 19367476 Review.
Cited by
-
Mechanistic insights into direct DNA and RNA strand transfer and dynamic protein exchange of SSB and RPA.Nucleic Acids Res. 2025 Jun 20;53(12):gkaf642. doi: 10.1093/nar/gkaf642. Nucleic Acids Res. 2025. PMID: 40598891 Free PMC article.
-
Single-Stranded DNA-Binding Proteins in the Archaea.Methods Mol Biol. 2021;2281:23-47. doi: 10.1007/978-1-0716-1290-3_2. Methods Mol Biol. 2021. PMID: 33847950 Review.
-
Förster resonance energy transfer and protein-induced fluorescence enhancement as synergetic multi-scale molecular rulers.Sci Rep. 2016 Sep 19;6:33257. doi: 10.1038/srep33257. Sci Rep. 2016. PMID: 27641327 Free PMC article.
-
Single-molecule FRET studies of the cooperative and non-cooperative binding kinetics of the bacteriophage T4 single-stranded DNA binding protein (gp32) to ssDNA lattices at replication fork junctions.Nucleic Acids Res. 2016 Dec 15;44(22):10691-10710. doi: 10.1093/nar/gkw863. Epub 2016 Sep 30. Nucleic Acids Res. 2016. PMID: 27694621 Free PMC article.
-
Prespacer processing and specific integration in a Type I-A CRISPR system.Nucleic Acids Res. 2018 Feb 16;46(3):1007-1020. doi: 10.1093/nar/gkx1232. Nucleic Acids Res. 2018. PMID: 29228332 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

