Iron Modulates Butyrate Production by a Child Gut Microbiota In Vitro
- PMID: 26578675
- PMCID: PMC4659462
- DOI: 10.1128/mBio.01453-15
Iron Modulates Butyrate Production by a Child Gut Microbiota In Vitro
Abstract
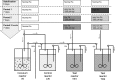
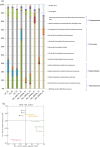
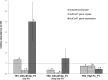
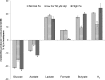
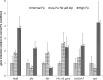
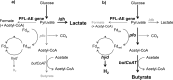
The aim of this study was to investigate the effect of iron (Fe) availability on butyrate production in the complex bacterial ecosystem of the human gut. Hence, different Fe availabilities were mimicked in an in vitro colonic fermentation model (the polyfermenter intestinal model called PolyFermS) inoculated with immobilized gut microbiota from a child and in batch cultures of the butyrate producer Roseburia intestinalis. Shifts in the microbial community (16S rRNA sequencing and quantitative PCR), metabolic activity (high-performance liquid chromatography), and expression of genes involved in butyrate production were assessed. In the PolyFermS, moderate Fe deficiency resulted in a 1.4-fold increase in butyrate production and a 5-fold increase in butyryl-coenzyme A (CoA):acetate CoA-transferase gene expression, while very strong Fe deficiency significantly decreased butyrate concentrations and butyrate-producing bacteria compared with the results under normal Fe conditions. Batch cultures of R. intestinalis grown in a low-Fe environment preferentially produced lactate and had reduced butyrate and hydrogen production, in parallel with upregulation of the lactate dehydrogenase gene and downregulation of the pyruvate:ferredoxin-oxidoreductase gene. In contrast, under high-Fe conditions, R. intestinalis cultures showed enhanced butyrate and hydrogen production, along with increased expression of the corresponding genes, compared with the results under normal-Fe conditions. Our data reveal the strong regulatory effect of Fe on gut microbiota butyrate producers and on the concentrations of butyrate, which contributes to the maintenance of host gut health.
Importance: Fe deficiency is one of the most common nutritional deficiencies worldwide and can be corrected by Fe supplementation. In this in vitro study, we show that environmental Fe concentrations in a continuous gut fermentation model closely mimicking a child's gut microbiota strongly affect the composition of the gut microbiome and its metabolic activity, particularly butyrate production. The differential expression of genes involved in the butyrate production pathway under different Fe conditions and the enzyme cofactor role of Fe explain the observed modulation of butyrate production. Our data reveal that the level of dietary Fe reaching the colon affects the microbiome, and its essential function of providing the host with beneficial butyrate.
Copyright © 2015 Dostal et al.
Figures
Similar articles
-
Cultivable butyrate-producing bacteria of elderly Japanese diagnosed with Alzheimer's disease.J Microbiol. 2018 Oct;56(10):760-771. doi: 10.1007/s12275-018-8297-7. Epub 2018 Aug 22. J Microbiol. 2018. PMID: 30136260
-
Low iron availability in continuous in vitro colonic fermentations induces strong dysbiosis of the child gut microbial consortium and a decrease in main metabolites.FEMS Microbiol Ecol. 2013 Jan;83(1):161-75. doi: 10.1111/j.1574-6941.2012.01461.x. Epub 2012 Aug 28. FEMS Microbiol Ecol. 2013. PMID: 22845175 Free PMC article.
-
Function and Phylogeny of Bacterial Butyryl Coenzyme A:Acetate Transferases and Their Diversity in the Proximal Colon of Swine.Appl Environ Microbiol. 2016 Oct 27;82(22):6788-6798. doi: 10.1128/AEM.02307-16. Print 2016 Nov 15. Appl Environ Microbiol. 2016. PMID: 27613689 Free PMC article.
-
Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine.FEMS Microbiol Lett. 2009 May;294(1):1-8. doi: 10.1111/j.1574-6968.2009.01514.x. Epub 2009 Feb 13. FEMS Microbiol Lett. 2009. PMID: 19222573 Review.
-
Formation of propionate and butyrate by the human colonic microbiota.Environ Microbiol. 2017 Jan;19(1):29-41. doi: 10.1111/1462-2920.13589. Epub 2016 Dec 8. Environ Microbiol. 2017. PMID: 27928878 Review.
Cited by
-
Species and individual rhinoceros affect the bacterial communities, metabolites, and nutrient composition in faeces from Southern black rhinoceros (Diceros bicornis minor) and Southern white rhinoceros (Ceratotherium simum simum) under managed care.J Anim Physiol Anim Nutr (Berl). 2022 Jan;106(1):181-193. doi: 10.1111/jpn.13520. Epub 2021 Mar 2. J Anim Physiol Anim Nutr (Berl). 2022. PMID: 33655648 Free PMC article.
-
Lachnospiraceae are emerging industrial biocatalysts and biotherapeutics.Front Bioeng Biotechnol. 2024 Jan 4;11:1324396. doi: 10.3389/fbioe.2023.1324396. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38239921 Free PMC article. Review.
-
Human matters in asthma: Considering the microbiome in pulmonary health.Front Pharmacol. 2022 Dec 2;13:1020133. doi: 10.3389/fphar.2022.1020133. eCollection 2022. Front Pharmacol. 2022. PMID: 36532717 Free PMC article. Review.
-
Stepwise Development of an in vitro Continuous Fermentation Model for the Murine Caecal Microbiota.Front Microbiol. 2019 May 29;10:1166. doi: 10.3389/fmicb.2019.01166. eCollection 2019. Front Microbiol. 2019. PMID: 31191488 Free PMC article.
-
Prebiotics Mediate Microbial Interactions in a Consortium of the Infant Gut Microbiome.Int J Mol Sci. 2017 Oct 4;18(10):2095. doi: 10.3390/ijms18102095. Int J Mol Sci. 2017. PMID: 28976925 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

