Feeding the brain and nurturing the mind: Linking nutrition and the gut microbiota to brain development
- PMID: 26578751
- PMCID: PMC4655552
- DOI: 10.1073/pnas.1511465112
Feeding the brain and nurturing the mind: Linking nutrition and the gut microbiota to brain development
Abstract
The human gut contains a microbial community composed of tens of trillions of organisms that normally assemble during the first 2-3 y of postnatal life. We propose that brain development needs to be viewed in the context of the developmental biology of this "microbial organ" and its capacity to metabolize the various diets we consume. We hypothesize that the persistent cognitive abnormalities seen in children with undernutrition are related in part to their persistent gut microbiota immaturity and that specific regions of the brain that normally exhibit persistent juvenile (neotenous) patterns of gene expression, including those critically involved in various higher cognitive functions such as the brain's default mode network, may be particularly vulnerable to the effects of microbiota immaturity in undernourished children. Furthermore, we postulate that understanding the interrelationships between microbiota and brain metabolism in childhood undernutrition could provide insights about responses to injury seen in adults. We discuss approaches that can be used to test these hypotheses, their ramifications for optimizing nutritional recommendations that promote healthy brain development and function, and the potential societal implications of this area of investigation.
Keywords: brain development; brain metabolism; childhood undernutrition; gut microbiota development.
Conflict of interest statement
Conflict of interest statement: J.I.G. is a cofounder of Matatu, Inc., a company characterizing the role of diet-by-microbiota interactions in animal health.
Figures
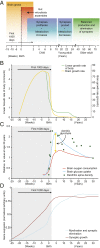
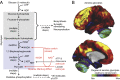

References
-
- Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

