High-resolution structures of the M2 channel from influenza A virus reveal dynamic pathways for proton stabilization and transduction
- PMID: 26578770
- PMCID: PMC4655559
- DOI: 10.1073/pnas.1518493112
High-resolution structures of the M2 channel from influenza A virus reveal dynamic pathways for proton stabilization and transduction
Erratum in
-
Correction for Thomaston et al., High-resolution structures of the M2 channel from influenza A virus reveal dynamic pathways for proton stabilization and transduction.Proc Natl Acad Sci U S A. 2016 May 3;113(18):E2548. doi: 10.1073/pnas.1605322113. Epub 2016 Apr 25. Proc Natl Acad Sci U S A. 2016. PMID: 27114529 Free PMC article. No abstract available.
Abstract
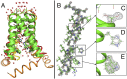
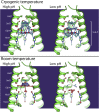
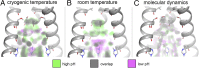
The matrix 2 (M2) protein from influenza A virus is a proton channel that uses His37 as a selectivity filter. Here we report high-resolution (1.10 Å) cryogenic crystallographic structures of the transmembrane domain of M2 at low and high pH. These structures reveal that waters within the pore form hydrogen-bonded networks or "water wires" spanning 17 Å from the channel entrance to His37. Pore-lining carbonyl groups are well situated to stabilize hydronium via second-shell interactions involving bridging water molecules. In addition, room temperature crystallographic structures indicate that water becomes increasingly fluid with increasing temperature and decreasing pH, despite the higher electrostatic field. Complementary molecular dynamics simulations reveal a collective switch of hydrogen bond orientations that can contribute to the directionality of proton flux as His37 is dynamically protonated and deprotonated in the conduction cycle.
Keywords: influenza M2 channel; membrane proteins; proton channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

