Role of HIV-1 matrix protein p17 variants in lymphoma pathogenesis
- PMID: 26578780
- PMCID: PMC4655530
- DOI: 10.1073/pnas.1514748112
Role of HIV-1 matrix protein p17 variants in lymphoma pathogenesis
Erratum in
-
Correction to Supporting Information for Dolcetti et al., Role of HIV-1 matrix protein p17 variants in lymphoma pathogenesis.Proc Natl Acad Sci U S A. 2015 Dec 15;112(50):E7033. doi: 10.1073/pnas.1522501112. Epub 2015 Nov 30. Proc Natl Acad Sci U S A. 2015. PMID: 26621736 Free PMC article. No abstract available.
Abstract
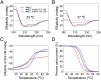
Although in decline after successful anti-HIV therapy, B-cell lymphomas are still elevated in HIV-1-seropositive (HIV+) persons, and the mechanisms are obscure. The HIV-1 matrix protein p17 persists in germinal centers long after HIV-1 drug suppression, and some p17 variants (vp17s) activate Akt signaling and promote growth of transformed B cells. Here we show that vp17s derived from four of five non-Hodgkin lymphoma (NHL) tissues from HIV+ subjects display potent B-cell growth-promoting activity. They are characterized by amino acid insertions at position 117-118 (Ala-Ala) or 125-126 (Gly-Asn or Gly-Gln-Ala-Asn-Gln-Asn) among some other mutations throughout the sequence. Identical dominant vp17s are found in both tumor and plasma. Three of seven plasma samples from an independent set of NHL cases manifested multiple Ala insertions at position 117-118, and one with the Ala-Ala profile also promoted B-cell growth and activated Akt signaling. Ultradeep pyrosequencing showed that vp17s with C-terminal insertions are more frequently detected in plasma of HIV+ subjects with than without NHL. Insertion of Ala-Ala at position 117-118 into reference p17 (refp17) was sufficient to confer B-cell growth-promoting activity. In contrast, refp17 bearing the Gly-Asn insertion at position 125-126 did not, suggesting that mutations not restricted to the C terminus can also account for this activity. Biophysical analysis revealed that the Ala-Ala insertion mutant is destabilized compared with refp17, whereas the Gly-Asn form is stabilized. This finding provides an avenue for further exploration of structure function relationships and new treatment strategies in combating HIV-1-related NHL.
Keywords: AIDS; B-cell clonogenicity; HIV-1 matrix protein p17; non-Hodgkin lymphoma; p17 variants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





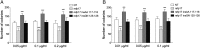
References
-
- Coté TR, et al. AIDS/Cancer Study Group Non-Hodgkin’s lymphoma among people with AIDS: Incidence, presentation and public health burden. Int J Cancer. 1997;73(5):645–650. - PubMed
-
- Gloghini A, Dolcetti R, Carbone A. Lymphomas occurring specifically in HIV-infected patients: From pathogenesis to pathology. Semin Cancer Biol. 2013;23(6):457–467. - PubMed
-
- Knowles DM. Etiology and pathogenesis of AIDS-related non-Hodgkin’s lymphoma. Hematol Oncol Clin North Am. 2003;17(3):785–820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

