Acquired equivalence associative learning in GTC epileptic patients: experimental and computational study
- PMID: 26578883
- PMCID: PMC4621864
- DOI: 10.3389/fncel.2015.00418
Acquired equivalence associative learning in GTC epileptic patients: experimental and computational study
Abstract
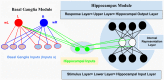
Previous cognitive behavioral studies based on Acquired Equivalence Associative learning Task (AEALT) showed a strong relation between hippocampus and basal ganglia in associative learning. However, experimental behavioral studies of patients with Generalized Tonic Clonic (GTC) epilepsy remained sparse. The aim of the present study is to integrate a classical behavioral cognitive analysis with a computational model approach to investigate cognitive associative learning impairments in patients with GTC epilepsy. We measured the accuracy of associative learning response performance in five GTC epileptic patients and five control subjects by using AEALT, all subjects were matched in age and gender. We ran the task using E-Prime, a neuropsychological software program, and SPSS for data statistical analysis. We tested whether GTC epileptic patients would have different learning performance than normal subjects, based on the degree and the location of impairment either in basal ganglia and/or hippocampus. With the number of patients that was available, our behavioral analysis showed no remarkable differences in learning performance of GTC patients as compared to their control subjects, both in the transfer and acquisition phases. In parallel, our simulation results confirmed strong connection and interaction between hippocampus and basal ganglia in our GTC and their control subjects. Nevertheless, the differences in neural firing rate of the connectionist model and weight update of basal ganglia were not significantly different between GTC and control subjects. Therefore, the behavioral analysis and the simulation data provided the same result, thus indicating that the computational model is likely to predict cognitive outcomes.
Keywords: acquired equivalence associative learning task; basal ganglia; cognitive impairment; connectionist model; generalized tonic clonic epilepsy; hippocampus.
Figures



References
-
- Barto A. G. (1995). Adaptive critics and the basal ganglia, in Models of Information Processing in the Basal Ganglia (pp. xii), eds Houk J. C., Davis J. L., Beiser D. G. (Cambridge, MA: MIT Press; ), 382.
-
- Berns G. S., Sejnowski T. J. (1996). How the basal ganglia make decisions, in The Neurobiology of Decision Making, eds Damasio A., Damasio H., Christen Y. (Berlin: Springer-Verlag; ), 101–113.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

