A Review of Transcranial Magnetic Stimulation and Multimodal Neuroimaging to Characterize Post-Stroke Neuroplasticity
- PMID: 26579069
- PMCID: PMC4625082
- DOI: 10.3389/fneur.2015.00226
A Review of Transcranial Magnetic Stimulation and Multimodal Neuroimaging to Characterize Post-Stroke Neuroplasticity
Abstract
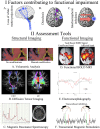
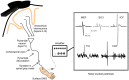
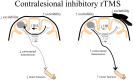
Following stroke, the brain undergoes various stages of recovery where the central nervous system can reorganize neural circuitry (neuroplasticity) both spontaneously and with the aid of behavioral rehabilitation and non-invasive brain stimulation. Multiple neuroimaging techniques can characterize common structural and functional stroke-related deficits, and importantly, help predict recovery of function. Diffusion tensor imaging (DTI) typically reveals increased overall diffusivity throughout the brain following stroke, and is capable of indexing the extent of white matter damage. Magnetic resonance spectroscopy (MRS) provides an index of metabolic changes in surviving neural tissue after stroke, serving as a marker of brain function. The neural correlates of altered brain activity after stroke have been demonstrated by abnormal activation of sensorimotor cortices during task performance, and at rest, using functional magnetic resonance imaging (fMRI). Electroencephalography (EEG) has been used to characterize motor dysfunction in terms of increased cortical amplitude in the sensorimotor regions when performing upper limb movement, indicating abnormally increased cognitive effort and planning in individuals with stroke. Transcranial magnetic stimulation (TMS) work reveals changes in ipsilesional and contralesional cortical excitability in the sensorimotor cortices. The severity of motor deficits indexed using TMS has been linked to the magnitude of activity imbalance between the sensorimotor cortices. In this paper, we will provide a narrative review of data from studies utilizing DTI, MRS, fMRI, EEG, and brain stimulation techniques focusing on TMS and its combination with uni- and multimodal neuroimaging methods to assess recovery after stroke. Approaches that delineate the best measures with which to predict or positively alter outcomes will be highlighted.
Keywords: diffusion tensor imaging; electroencephalography; functional MRI; magnetic resonance spectroscopy; multimodal neuroimaging; sensorimotor recovery; stroke; transcranial magnetic stimulation.
Figures

References
-
- Boyd LA, Winstein CJ. Impact of explicit information on implicit motor-sequence learning following middle cerebral artery stroke. Phys Ther (2003) 83(11):976–89. - PubMed
-
- Taub E, Miller NE, Novack TA, Cook EW, Fleming WC, Nepomuceno CS, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil (1993) 74:347–54. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

