Effector and Central Memory Poly-Functional CD4(+) and CD8(+) T Cells are Boosted upon ZOSTAVAX(®) Vaccination
- PMID: 26579128
- PMCID: PMC4629102
- DOI: 10.3389/fimmu.2015.00553
Effector and Central Memory Poly-Functional CD4(+) and CD8(+) T Cells are Boosted upon ZOSTAVAX(®) Vaccination
Abstract
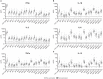
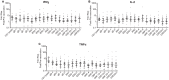
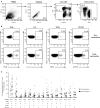
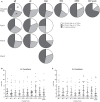
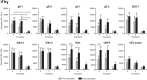
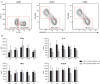
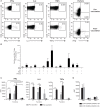
ZOSTAVAX(®) is a live attenuated varicella-zoster virus (VZV) vaccine that is licensed for the protection of individuals ≥50 years against shingles and its most common complication, postherpetic neuralgia. While IFNγ responses increase upon vaccination, the quality of the T cell response has not been elucidated. By using polychromatic flow cytometry, we characterized the breadth, magnitude, and quality of ex vivo CD4(+) and CD8(+) T cell responses induced 3-4 weeks after ZOSTAVAX vaccination of healthy adults. We show, for the first time that the highest frequencies of VZV-specific CD4(+) T cells were poly-functional CD154(+)IFNγ(+)IL-2(+)TNFα(+) cells, which were boosted upon vaccination. The CD4(+) T cells were broadly reactive to several VZV proteins, with immediate early (IE) 63 ranking the highest among them in the fold rise of poly-functional cells, followed by IE62, gB, open reading frame (ORF) 9, and gE. We identified a novel poly-functional ORF9-specific CD8(+) T cell population in 62% of the subjects, and these were boosted upon vaccination. Poly-functional CD4(+) and CD8(+) T cells produced significantly higher levels of IFNγ, IL-2, and TNFα compared to mono-functional cells. After vaccination, a boost in the expression of IFNγ by poly-functional IE63- and ORF9-specific CD4(+) T cells and IFNγ, IL-2, and TNFα by ORF9-specific poly-functional CD8(+) T cells was observed. Responding poly-functional T cells exhibited both effector (CCR7(-)CD45RA(-)CD45RO(+)), and central (CCR7(+)CD45RA(-)CD45RO(+)) memory phenotypes, which expressed comparable levels of cytokines. Altogether, our studies demonstrate that a boost in memory poly-functional CD4(+) T cells and ORF9-specific CD8(+) T cells may contribute toward ZOSTAVAX efficacy.
Keywords: VZV antigens; ZOSTAVAX; flow cytometry; memory response; poly-functional T cells.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

