Evolutionary transgenomics: prospects and challenges
- PMID: 26579137
- PMCID: PMC4620933
- DOI: 10.3389/fpls.2015.00858
Evolutionary transgenomics: prospects and challenges
Abstract
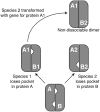
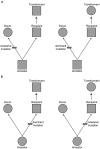
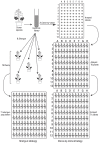
Many advances in our understanding of the genetic basis of species differences have arisen from transformation experiments, which allow us to study the effect of genes from one species (the donor) when placed in the genetic background of another species (the recipient). Such interspecies transformation experiments are usually focused on candidate genes - genes that, based on work in model systems, are suspected to be responsible for certain phenotypic differences between the donor and recipient species. We suggest that the high efficiency of transformation in a few plant species, most notably Arabidopsis thaliana, combined with the small size of typical plant genes and their cis-regulatory regions allow implementation of a screening strategy that does not depend upon a priori candidate gene identification. This approach, transgenomics, entails moving many large genomic inserts of a donor species into the wild type background of a recipient species and then screening for dominant phenotypic effects. As a proof of concept, we recently conducted a transgenomic screen that analyzed more than 1100 random, large genomic inserts of the Alabama gladecress Leavenworthia alabamica for dominant phenotypic effects in the A. thaliana background. This screen identified one insert that shortens fruit and decreases A. thaliana fertility. In this paper we discuss the principles of transgenomic screens and suggest methods to help minimize the frequencies of false positive and false negative results. We argue that, because transgenomics avoids committing in advance to candidate genes it has the potential to help us identify truly novel genes or cryptic functions of known genes. Given the valuable knowledge that is likely to be gained, we believe the time is ripe for the plant evolutionary community to invest in transgenomic screens, at least in the mustard family Brassicaceae where many species are amenable to efficient transformation.
Keywords: developmental system drift; evo-devo; evolution; genetic screens; speciation genes; transformation; transgenomics.
Figures

Similar articles
-
Interspecies Gene Transfer as a Method for Understanding the Genetic Basis for Evolutionary Change: Progress, Pitfalls, and Prospects.Front Plant Sci. 2015 Dec 22;6:1135. doi: 10.3389/fpls.2015.01135. eCollection 2015. Front Plant Sci. 2015. PMID: 26734038 Free PMC article.
-
An assessment of transgenomics as a tool for identifying genes involved in the evolutionary differentiation of closely related plant species.New Phytol. 2012 Jan;193(2):494-503. doi: 10.1111/j.1469-8137.2011.03949.x. Epub 2011 Nov 11. New Phytol. 2012. PMID: 22077724 Free PMC article.
-
Lepidium as a model system for studying the evolution of fruit development in Brassicaceae.J Exp Bot. 2009;60(5):1503-13. doi: 10.1093/jxb/ern304. Epub 2008 Dec 3. J Exp Bot. 2009. PMID: 19052256 Review.
-
The locus of evolution: evo devo and the genetics of adaptation.Evolution. 2007 May;61(5):995-1016. doi: 10.1111/j.1558-5646.2007.00105.x. Evolution. 2007. PMID: 17492956 Review.
-
From phenotypic to molecular polymorphisms involved in naturally occurring variation of plant development.Int J Dev Biol. 2005;49(5-6):717-32. doi: 10.1387/ijdb.051994ca. Int J Dev Biol. 2005. PMID: 16096977 Review.
Cited by
-
An assessment of transgenomics as a tool for gene discovery in Populus euphratica Oliv.Plant Mol Biol. 2018 Aug;97(6):525-535. doi: 10.1007/s11103-018-0755-4. Epub 2018 Jul 26. Plant Mol Biol. 2018. Retraction in: Plant Mol Biol. 2018 Dec;98(6):579. doi: 10.1007/s11103-018-0798-6. PMID: 30051252 Retracted.
-
Nitrate/ammonium-responsive microRNA-mRNA regulatory networks affect root system architecture in Populus × canescens.BMC Plant Biol. 2022 Mar 4;22(1):96. doi: 10.1186/s12870-022-03482-3. BMC Plant Biol. 2022. PMID: 35246022 Free PMC article.
-
Interspecies Gene Transfer as a Method for Understanding the Genetic Basis for Evolutionary Change: Progress, Pitfalls, and Prospects.Front Plant Sci. 2015 Dec 22;6:1135. doi: 10.3389/fpls.2015.01135. eCollection 2015. Front Plant Sci. 2015. PMID: 26734038 Free PMC article.
References
-
- Baum D. A. (2002). “Identifying the genetic causes of phenotypic evolution: a review of experimental strategies,” in Developmental Genetics and Plant Evolution, eds Cronk Q. C. B., Bateman R. M., Hawkins J. A. (London: Taylor & Francis; ), 493–507.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

