Salinity tolerance in plants. Quantitative approach to ion transport starting from halophytes and stepping to genetic and protein engineering for manipulating ion fluxes
- PMID: 26579140
- PMCID: PMC4621421
- DOI: 10.3389/fpls.2015.00873
Salinity tolerance in plants. Quantitative approach to ion transport starting from halophytes and stepping to genetic and protein engineering for manipulating ion fluxes
Abstract
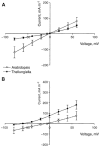
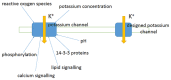
Ion transport is the fundamental factor determining salinity tolerance in plants. The Review starts from differences in ion transport between salt tolerant halophytes and salt-sensitive plants with an emphasis on transport of potassium and sodium via plasma membranes. The comparison provides introductory information for increasing salinity tolerance. Effects of salt stress on ion transport properties of membranes show huge opportunities for manipulating ion fluxes. Further steps require knowledge about mechanisms of ion transport and individual genes of ion transport proteins. Initially, the Review describes methods to measure ion fluxes, the independent set of techniques ensures robust and reliable basement for quantitative approach. The Review briefly summarizes current data concerning Na(+) and K(+) concentrations in cells, refers to primary thermodynamics of ion transport and gives special attention to individual ion channels and transporters. Simplified scheme of a plant cell with known transport systems at the plasma membrane and tonoplast helps to imagine the complexity of ion transport and allows choosing specific transporters for modulating ion transport. The complexity is enhanced by the influence of cell size and cell wall on ion transport. Special attention is given to ion transporters and to potassium and sodium transport by HKT, HAK, NHX, and SOS1 proteins. Comparison between non-selective cation channels and ion transporters reveals potential importance of ion transporters and the balance between the two pathways of ion transport. Further on the Review describes in detail several successful attempts to overexpress or knockout ion transporters for changing salinity tolerance. Future perspectives are questioned with more attention given to promising candidate ion channels and transporters for altered expression. Potential direction of increasing salinity tolerance by modifying ion channels and transporters using single point mutations is discussed and questioned. An alternative approach from synthetic biology is to create new regulation networks using novel transport proteins with desired properties for transforming agricultural crops. The approach had not been widely used earlier; it leads also to theoretical and pure scientific aspects of protein chemistry, structure-function relations of membrane proteins, systems biology and physiology of stress and ion homeostasis. Summarizing, several potential ways are aimed at required increase in salinity tolerance of plants of interest.
Keywords: halophyte; ion channel; ion fluxes; ion transporter; protein engineering; salinity tolerance; synthetic biology; systems biology.
Figures








References
-
- Akhromeeva T. S., Kurdyumov S. P., Malinetskii G. G., Samarskii A. A. (1989). Nonstationary dissipative structures and diffusion-induced chaos in nonlinear media. Physics. Rep. 176 5–6. 10.1016/0370-1573(89)90001-X - DOI
-
- Akhromeeva T. S., Kurdyumov S. P., Malinetskij G. G., Samarskij A. A. (1992). Nonstationary Structures and Diffusion Chaos (Nestatsionarnye Struktury i Diffuznyj Khaos). Moskva: Nauka Publisher; 544.
-
- Alemán F., Caballero F., Ródenas R., Rivero R. M., Martínez V., Rubio F. (2014). The F130S point mutation in the Arabidopsis high-affinity K+ transporter AtHAK5 increases K+ over Na+ and Cs+ selectivity and confers Na+ and Cs+ tolerance to yeast under heterologous expression. Front. Plant Sci. 5:430 10.3389/fpls.2014.00430 - DOI - PMC - PubMed
-
- Alemán F., Nieves-Cordones M., Martínez V., Rubio F. (2009). Potassium/sodium steady-state homeostasis in Thellungiella halophila and Arabidopsis thaliana under long-term salinity conditions. Plant Sci. 176 768–774. 10.1016/j.plantsci.2009.02.020 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

