DNA maintenance in plastids and mitochondria of plants
- PMID: 26579143
- PMCID: PMC4624840
- DOI: 10.3389/fpls.2015.00883
DNA maintenance in plastids and mitochondria of plants
Abstract
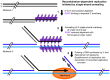
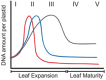
The DNA molecules in plastids and mitochondria of plants have been studied for over 40 years. Here, we review the data on the circular or linear form, replication, repair, and persistence of the organellar DNA (orgDNA) in plants. The bacterial origin of orgDNA appears to have profoundly influenced ideas about the properties of chromosomal DNA molecules in these organelles to the point of dismissing data inconsistent with ideas from the 1970s. When found at all, circular genome-sized molecules comprise a few percent of orgDNA. In cells active in orgDNA replication, most orgDNA is found as linear and branched-linear forms larger than the size of the genome, likely a consequence of a virus-like DNA replication mechanism. In contrast to the stable chromosomal DNA molecules in bacteria and the plant nucleus, the molecular integrity of orgDNA declines during leaf development at a rate that varies among plant species. This decline is attributed to degradation of damaged-but-not-repaired molecules, with a proposed repair cost-saving benefit most evident in grasses. All orgDNA maintenance activities are proposed to occur on the nucleoid tethered to organellar membranes by developmentally-regulated proteins.
Keywords: DNA recombination; DNA repair; DNA replication; chloroplast DNA; organellar DNA.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

