Effect of temperature on the pathogenesis, accumulation of viral and satellite RNAs and on plant proteome in peanut stunt virus and satellite RNA-infected plants
- PMID: 26579153
- PMCID: PMC4625170
- DOI: 10.3389/fpls.2015.00903
Effect of temperature on the pathogenesis, accumulation of viral and satellite RNAs and on plant proteome in peanut stunt virus and satellite RNA-infected plants
Erratum in
-
Corrigendum: Effect of temperature on the pathogenesis, accumulation of viral and satellite RNAs and on plant proteome in peanut stunt virus and satellite RNA-infected plants.Front Plant Sci. 2016 Jun 7;7:839. doi: 10.3389/fpls.2016.00839. eCollection 2016. Front Plant Sci. 2016. PMID: 27375674 Free PMC article.
Abstract
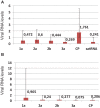
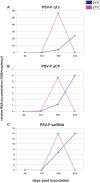
Temperature is an important environmental factor influencing plant development in natural and diseased conditions. The growth rate of plants grown at C27°C is more rapid than for plants grown at 21°C. Thus, temperature affects the rate of pathogenesis progression in individual plants. We have analyzed the effect of temperature conditions (either 21°C or 27°C during the day) on the accumulation rate of the virus and satellite RNA (satRNA) in Nicotiana benthamiana plants infected by peanut stunt virus (PSV) with and without its satRNA, at four time points. In addition, we extracted proteins from PSV and PSV plus satRNA-infected plants harvested at 21 dpi, when disease symptoms began to appear on plants grown at 21°C and were well developed on those grown at 27°C, to assess the proteome profile in infected plants compared to mock-inoculated plants grown at these two temperatures, using 2D-gel electrophoresis and mass spectrometry approaches. The accumulation rate of the viral RNAs and satRNA was more rapid at 27°C at the beginning of the infection and then rapidly decreased in PSV-infected plants. At 21 dpi, PSV and satRNA accumulation was higher at 21°C and had a tendency to increase further. In all studied plants grown at 27°C, we observed a significant drop in the identified proteins participating in photosynthesis and carbohydrate metabolism at the proteome level, in comparison to plants maintained at 21°C. On the other hand, the proteins involved in protein metabolic processes were all more abundant in plants grown at 27°C. This was especially evident when PSV-infected plants were analyzed, where increase in abundance of proteins involved in protein synthesis, degradation, and folding was revealed. In mock-inoculated and PSV-infected plants we found an increase in abundance of the majority of stress-related differently-regulated proteins and those associated with protein metabolism. In contrast, in PSV plus satRNA-infected plants the shift in the temperature barely increased the level of stress-related proteins.
Keywords: DIGE; cucumovirus; leaf proteome; plant defense; plant proteomics; plant-virus interactions; satellite RNA; temperature change.
Figures

Similar articles
-
How can plant virus satellite RNAs alter the effects of plant virus infection? A study of the changes in the Nicotiana benthamiana proteome after infection by peanut stunt virus in the presence or absence of its satellite RNA.Proteomics. 2013 Jul;13(14):2162-75. doi: 10.1002/pmic.201200056. Epub 2013 Jun 10. Proteomics. 2013. PMID: 23580405
-
Peanut Stunt Virus and Its Satellite RNA Trigger Changes in Phosphorylation in N. benthamiana Infected Plants at the Early Stage of the Infection.Int J Mol Sci. 2018 Oct 18;19(10):3223. doi: 10.3390/ijms19103223. Int J Mol Sci. 2018. PMID: 30340407 Free PMC article.
-
The Defense Response of Nicotiana benthamiana to Peanut Stunt Virus Infection in the Presence of Symptom Exacerbating Satellite RNA.Viruses. 2018 Aug 23;10(9):449. doi: 10.3390/v10090449. Viruses. 2018. PMID: 30142955 Free PMC article.
-
Symptom-modulating properties of peanut stunt virus satellite RNA sequence variants.Mol Plant Microbe Interact. 1991 May-Jun;4(3):268-75. doi: 10.1094/mpmi-4-268. Mol Plant Microbe Interact. 1991. PMID: 1718510
-
Multiple cellular compartments engagement in Nicotiana benthamiana-peanut stunt virus-satRNA interactions revealed by systems biology approach.Plant Cell Rep. 2021 Jul;40(7):1247-1267. doi: 10.1007/s00299-021-02706-4. Epub 2021 May 24. Plant Cell Rep. 2021. PMID: 34028582 Free PMC article.
Cited by
-
Interactive Responses of Potato (Solanum tuberosum L.) Plants to Heat Stress and Infection With Potato Virus Y.Front Microbiol. 2018 Oct 30;9:2582. doi: 10.3389/fmicb.2018.02582. eCollection 2018. Front Microbiol. 2018. PMID: 30425697 Free PMC article.
-
Tannins and copper sulphate as antimicrobial agents to prevent contamination of Posidonia oceanica seedling culture for restoration purposes.Front Plant Sci. 2024 Nov 25;15:1433358. doi: 10.3389/fpls.2024.1433358. eCollection 2024. Front Plant Sci. 2024. PMID: 39654965 Free PMC article.
-
Exploring the Diversity of Mechanisms Associated With Plant Tolerance to Virus Infection.Front Plant Sci. 2018 Nov 2;9:1575. doi: 10.3389/fpls.2018.01575. eCollection 2018. Front Plant Sci. 2018. PMID: 30450108 Free PMC article. Review.
-
Light Intensity Modulates the Efficiency of Virus Seed Transmission through Modifications of Plant Tolerance.Plants (Basel). 2019 Aug 27;8(9):304. doi: 10.3390/plants8090304. Plants (Basel). 2019. PMID: 31461899 Free PMC article.
-
Assessment of the Efficacy and Mode of Action of Benzo(1,2,3)-Thiadiazole-7-Carbothioic Acid S-Methyl Ester (BTH) and Its Derivatives in Plant Protection Against Viral Disease.Int J Mol Sci. 2019 Mar 30;20(7):1598. doi: 10.3390/ijms20071598. Int J Mol Sci. 2019. PMID: 30935036 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

