Dynamic epigenetic states of maize centromeres
- PMID: 26579154
- PMCID: PMC4620398
- DOI: 10.3389/fpls.2015.00904
Dynamic epigenetic states of maize centromeres
Abstract
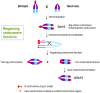
The centromere is a specialized chromosomal region identified as the major constriction, upon which the kinetochore complex is formed, ensuring accurate chromosome orientation and segregation during cell division. The rapid evolution of centromere DNA sequence and the conserved centromere function are two contradictory aspects of centromere biology. Indeed, the sole presence of genetic sequence is not sufficient for centromere formation. Various dicentric chromosomes with one inactive centromere have been recognized. It has also been found that de novo centromere formation is common on fragments in which centromeric DNA sequences are lost. Epigenetic factors play important roles in centromeric chromatin assembly and maintenance. Non-disjunction of the supernumerary B chromosome centromere is independent of centromere function, but centromere pairing during early prophase of meiosis I requires an active centromere. This review discusses recent studies in maize about genetic and epigenetic elements regulating formation and maintenance of centromere chromatin, as well as centromere behavior in meiosis.
Keywords: centromere inactivation; centromere pairing; de novo centromere; epigenetics; maize; neocentromere; non-disjunction.
Figures





Similar articles
-
Sequential de novo centromere formation and inactivation on a chromosomal fragment in maize.Proc Natl Acad Sci U S A. 2015 Mar 17;112(11):E1263-71. doi: 10.1073/pnas.1418248112. Epub 2015 Mar 2. Proc Natl Acad Sci U S A. 2015. PMID: 25733907 Free PMC article.
-
Switching the centromeres on and off: epigenetic chromatin alterations provide plasticity in centromere activity stabilizing aberrant dicentric chromosomes.Biochem Soc Trans. 2013 Dec;41(6):1648-53. doi: 10.1042/BST20130136. Biochem Soc Trans. 2013. PMID: 24256269 Review.
-
Dicentric chromosome formation and epigenetics of centromere formation in plants.J Genet Genomics. 2012 Mar 20;39(3):125-30. doi: 10.1016/j.jgg.2012.01.006. Epub 2012 Feb 14. J Genet Genomics. 2012. PMID: 22464471 Review.
-
Recurrent establishment of de novo centromeres in the pericentromeric region of maize chromosome 3.Chromosome Res. 2017 Oct;25(3-4):299-311. doi: 10.1007/s10577-017-9564-x. Epub 2017 Aug 22. Chromosome Res. 2017. PMID: 28831743
-
Centromere pairing in early meiotic prophase requires active centromeres and precedes installation of the synaptonemal complex in maize.Plant Cell. 2013 Oct;25(10):3900-9. doi: 10.1105/tpc.113.117846. Epub 2013 Oct 18. Plant Cell. 2013. PMID: 24143803 Free PMC article.
Cited by
-
Different Families of Retrotransposons and DNA Transposons Are Actively Transcribed and May Have Transposed Recently in Physcomitrium (Physcomitrella) patens.Front Plant Sci. 2020 Aug 19;11:1274. doi: 10.3389/fpls.2020.01274. eCollection 2020. Front Plant Sci. 2020. PMID: 32973835 Free PMC article.
-
The Behavior of the Maize B Chromosome and Centromere.Genes (Basel). 2018 Oct 1;9(10):476. doi: 10.3390/genes9100476. Genes (Basel). 2018. PMID: 30275397 Free PMC article. Review.
-
Plasticity of parental CENH3 incorporation into the centromeres in wheat × barley F1 hybrids.Front Plant Sci. 2024 Jan 19;15:1324817. doi: 10.3389/fpls.2024.1324817. eCollection 2024. Front Plant Sci. 2024. PMID: 38313805 Free PMC article.
-
Cenh3: An Emerging Player in Haploid Induction Technology.Front Plant Sci. 2016 Apr 12;7:357. doi: 10.3389/fpls.2016.00357. eCollection 2016. Front Plant Sci. 2016. PMID: 27148276 Free PMC article. Review.
-
Centromere histone H3- and phospholipase-mediated haploid induction in plants.Plant Methods. 2019 Apr 26;15:42. doi: 10.1186/s13007-019-0429-5. eCollection 2019. Plant Methods. 2019. PMID: 31057661 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

